Application of nuciferine and lotus leaf extract for preparing drug for treating atrophic gastritis and/or blocking transformation of gastritis cancer
A technology of atrophic gastritis and lotus leaf extract, which is applied in the field of natural small molecule compounds of traditional Chinese medicine and traditional Chinese medicine extracts, and can solve the problems of reports of transformational therapeutic effects of digestive tract inflammation and cancer that have not been found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
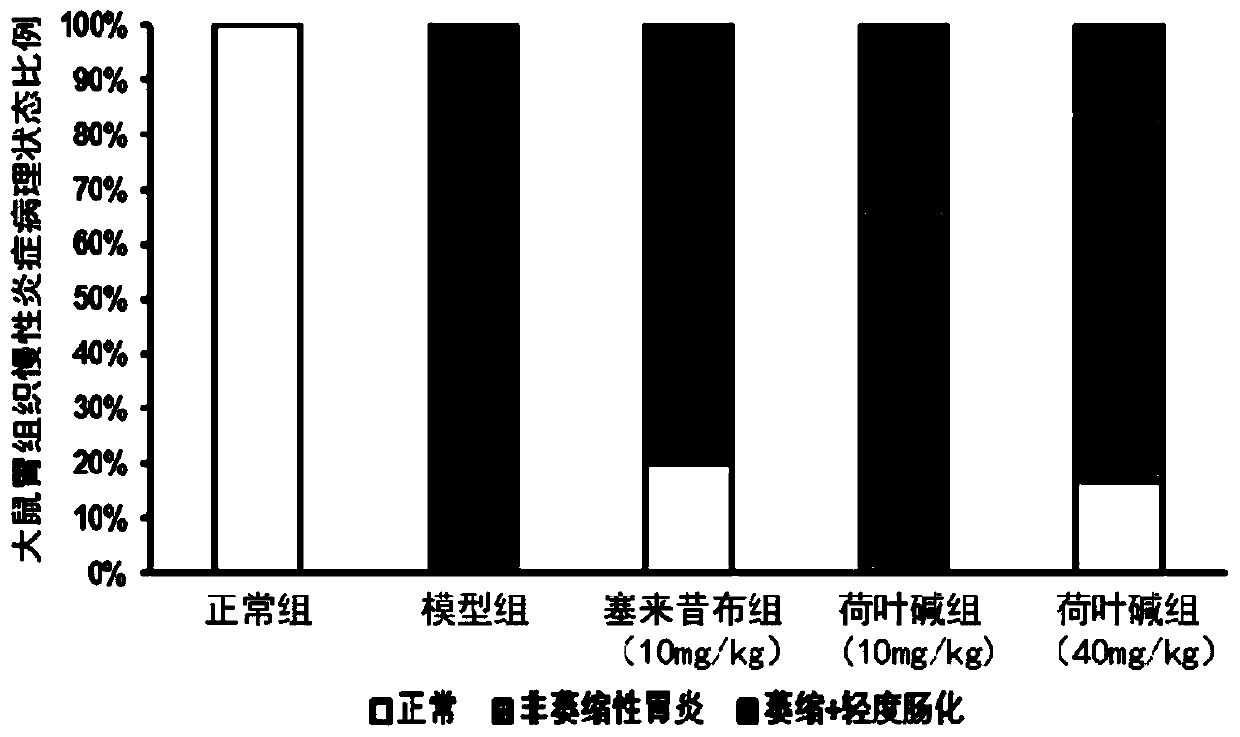
[0054] Example 1: Nuciferine has the effect of treating chronic atrophic gastritis rats with intestinal metaplasia
Embodiment approach
[0055] Implementation scheme: The experimental animals were from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. Select 30 specific pathogen free (SPF) grade 200±20g SD rats, half male and half male, and randomly divide them into 5 groups: normal control group, chronic atrophic gastritis (CAG) model group, The positive drug celecoxib intervention group and the two-dose intervention group of nuciferine, 6 rats in each group. Nuciferine was purchased from Chengdu Ruifensi Biotechnology Co., Ltd., with a specification of 5g and a purity of over 98%. Celecoxib was purchased from Beijing Bailingwei Technology Co., Ltd., brand J&K, with a specification of 1 g and a purity of over 98%. Sodium deoxycholate was purchased from Amresco, USA, with a specification of 100 g. Rats in model group, celecoxib intervention group and nuciferine intervention group were given sodium deoxycholate solution, ammonia water and ethanol to induce chronic atrophic gastritis in rats. Dail...
Embodiment 2
[0066] Example 2: Nuciferine and lotus leaf extract have a protective effect on gastric mucosal damage
[0067] Experimental plan: The experimental animals were from Beijing Best Biotechnology Co., Ltd., 30 SD rats of Specific pathogen Free (SPF) grade 200±20g were selected, male, and randomly divided into 5 groups: normal control group, gastric mucosa Injury model group, low-dose nuciferine group, high-dose nuciferine group and lotus leaf extract group. Nuciferine low-dose group, Nuciferine high-dose group and lotus leaf extract group were administered intragastrically for 14 consecutive days, once a day. Nuciferine was purchased from Chengdu Ruifensi Biotechnology Co., Ltd., with a specification of 5g and a purity of over 98%. The lotus leaf extract was purchased from Hebei Chenguang Biotechnology Group Handan Co., Ltd., specification: 1kg, and the content of nuciferine was above 0.4%. After the rats in the low-dose nuciferine group, the high-dose nuciferine group and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com