Preparation method and application of a battery catalyst
A catalyst and battery technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve problems such as poor cycle performance, high charge-discharge overpotential, and low energy conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] In one embodiment, a method for preparing a battery catalyst comprises the following steps:
[0038] A method for preparing a battery catalyst, comprising the steps of:
[0039] S1. Using noble metal salts and magnesium salts to prepare a mixed solution containing noble metals and magnesium ions;
[0040] S2. Adding a complexing agent to the mixed solution containing noble metals and magnesium ions to prepare a complexing solution;
[0041] S3, evaporating the volatile solvent in the complex solution containing noble metals and magnesium ions to obtain a gel containing noble metals and magnesium ions;
[0042] S4, heat treating the gel to obtain a foamy precursor, and performing a second heat treatment on the precursor to obtain a noble metal / magnesia composite powder or a noble metal oxide / magnesia composite powder;
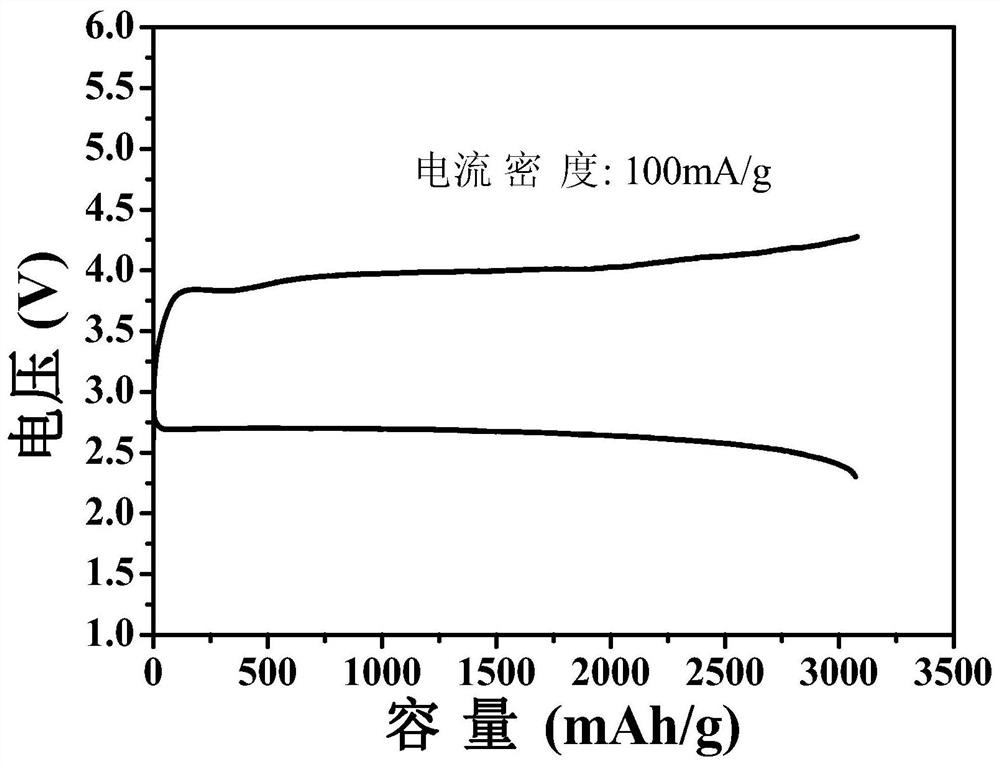
[0043] S5. Adding the noble metal / magnesia composite powder or the noble metal oxide / magnesia composite powder into the acid solution for etching, and ...
Embodiment 1
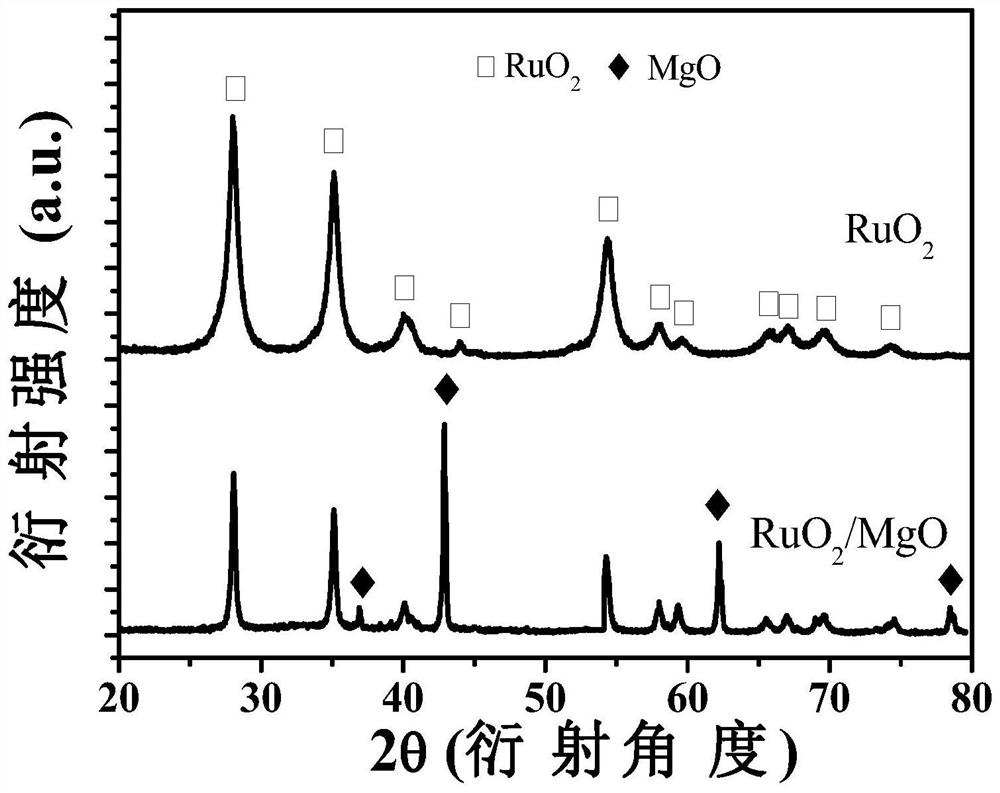
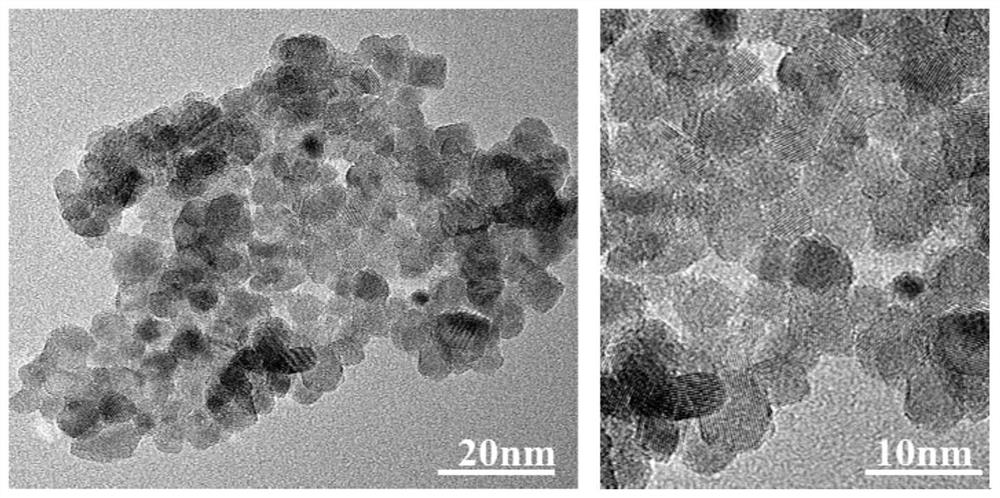
[0059] Ultrafine monodisperse RuO 2 Preparation:
[0060] Dissolve ruthenium chloride hydrate (or ruthenium salts such as ruthenium acetate) in deionized water or organic solvents (ethanol, ether, acetone, chloroform, etc.) to form solution A, weigh an appropriate amount of magnesium acetate and dissolve it in solution A , forming solution B. Wherein, the molar ratio of magnesium to ruthenium ions is 0.5:1-1:10. Weigh an appropriate amount of sucrose (or citric acid, or ethylenediaminetetraacetic acid, or glycine, or an organic complexing agent such as polyvinylpyrrolidone) and dissolve it in solution B to form solution C. Wherein, the ratio of sucrose (or citric acid, or ethylenediaminetetraacetic acid, or glycine, or polyvinylpyrrolidone) to the total molar concentration of ruthenium and magnesium ions is 1:0.5-1:10. Put solution C on a magnetic heating stirrer, heat to evaporate water (or organic solvent), and obtain gel D. Put D in a forced air drying oven at 200 degre...
Embodiment 2
[0062] Preparation method of ultrafine monodisperse PdO:
[0063] Dissolve palladium chloride (or palladium salts such as palladium nitrate) in deionized water or organic solvents (ethanol, ether, acetone, etc.) to form solution A, weigh an appropriate amount of magnesium acetate and dissolve it in solution A to form solution B . Wherein, the molar ratio of magnesium to palladium ions is 0.5:1-1:10. Weigh an appropriate amount of sucrose (or citric acid, or ethylenediaminetetraacetic acid, or glycine, or polyvinylpyrrolidone) and dissolve it in solution B to form solution C. Wherein, the ratio of sucrose (or citric acid, or ethylenediaminetetraacetic acid, or glycine, or polyvinylpyrrolidone) to the total molar concentration of palladium and magnesium ions is 1:0.5-1:10. Put solution C on a magnetic heating stirrer, heat to evaporate water (or organic solvent), and obtain gel D. Put D in a forced air drying oven at 200 degrees Celsius for 4-24 hours to obtain foamy precurso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com