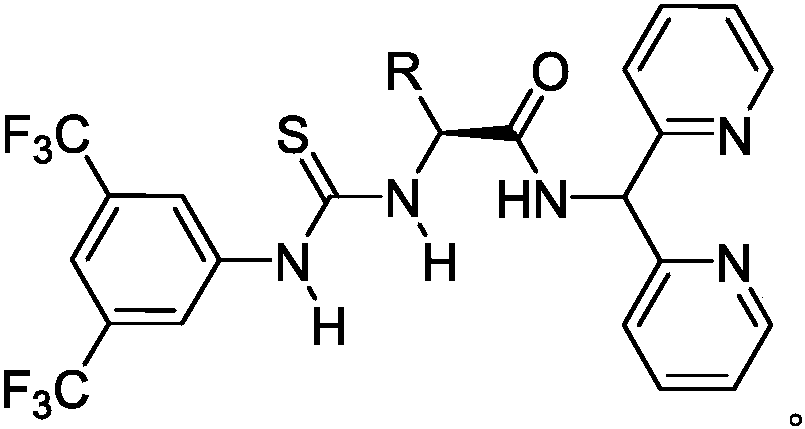
Thiourea-dipicolinamide catalyst as well as preparation method and application thereof
A technology of aminobipyridine amide and pyridine amide, which is applied in the field of asymmetric catalysis, and achieves the effects of cheap chiral source, wide application range of substrate and easy access to chiral source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
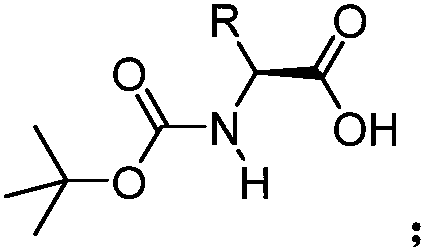
[0046] Compound 3: Synthesis of (S)-aminobipyridinamide intermediate protected by tert-butoxycarbonyl group:
[0047]
[0048] Synthesis of compound 3a: Add dipyridylmethylamine (0.5g, 2.7mmol), 4-Dimethylaminopyridine (DMAP, 65.8mg, 0.54mmol, 0.2 equivalents) and 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI, 0.62g, 3.2mmol, 1.2 equivalents). After the mixture was stirred at room temperature for 12 hours, the organic phase was extracted with ethyl acetate and washed with saturated brine. The combined organic phases were dried and concentrated, separated by column chromatography, and eluted with dichloromethane / methanol 19:1 to obtain N-tert-butoxycarbonyl-(L)-alaninamide dipyridinecarboxamide intermediate 3a, Yield 82% (0.79 g, 2.2 mmol).
[0049] Synthesis of compound 3b: To N-tert-butoxycarbonyl-L-phenylalanine 1a (0.86g, 3.2mmol, 1.2 equivalents) in dichloromethane solution, dipyridylmethylamine (0.5g, 2.7mmol) was added successively , 4-dimethyla...
Embodiment 2
[0053] Compound 6: Synthesis of thiourea-dipyridinamide catalyst:
[0054]
[0055] Synthesis of compound 6a: To a solution of N-tert-butoxycarbonyl-(L)-alaninamide dipyridinecarboxamide intermediate (0.79 g, 2.2 mmol) in dichloromethane (22 mL) was added trifluoroacetic acid (2.2 mL) . The reaction mixture was de-tert-butoxycarbonylated at room temperature for 6 hours. After the completion of the reaction was tracked by thin layer chromatography (TLC), the pH value was adjusted to 10-12 with ammonia water in a zero-degree ice bath. The residue was extracted with ethyl acetate and dried over anhydrous magnesium sulfate. After the organic phase was concentrated, it was dissolved in tetrahydrofuran, 3,5-bis(trifluoromethyl)phenylisothiocyanate (0.71g, 2.6mmol) was added, concentrated after 12 hours of reaction, separated by column chromatography, dichloromethane / methanol 9:1 to obtain compound 6a. White solid, 67% yield (0.77 g, 1.5 mmol).
[0056] Synthesis of compound...
Embodiment 3
[0064] Catalytic effect experiment: the thiourea-bipyridine amide chiral catalyst 6a-6d of the present invention is applied in an asymmetric reaction, such as compound 6d in the asymmetric Michael addition reaction of nitrostyrene and diethyl malonate application, the process is as follows:
[0065] Diethyl malonate (24.0 mg, 0.15 mmol), compound 6d (5.7 mg, 0.01 mmol, 10 mol%), and ketone trifluoromethanesulfonate were sequentially added to a toluene solution of a nitrostyrene derivative (0.1 mmol) (1.8 mg, 0.005 mmol, 5 mol%). The reaction mixture was reacted at room temperature. After the completion of the reaction was traced by thin layer chromatography, it was separated by column chromatography and eluted with petroleum ether / ethyl acetate 9:1 to obtain the Michael addition product.
[0066] The reaction formula is as follows:
[0067]
[0068] The catalytic effect is shown in the table below:
[0069] Table 2
[0070]
[0071] As can be seen from the above tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com