Catalyst for preparing low carbon aromatic hydrocarbon by hydrodealkylation of C10+ heavyweight aromatic hydrocarbon and preparation method of catalyst
A technology for hydrodealkylation and low-carbon aromatics, applied in physical/chemical process catalysts, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems of poor processing capacity of heavy aromatics, reduce production costs, and improve utilization rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
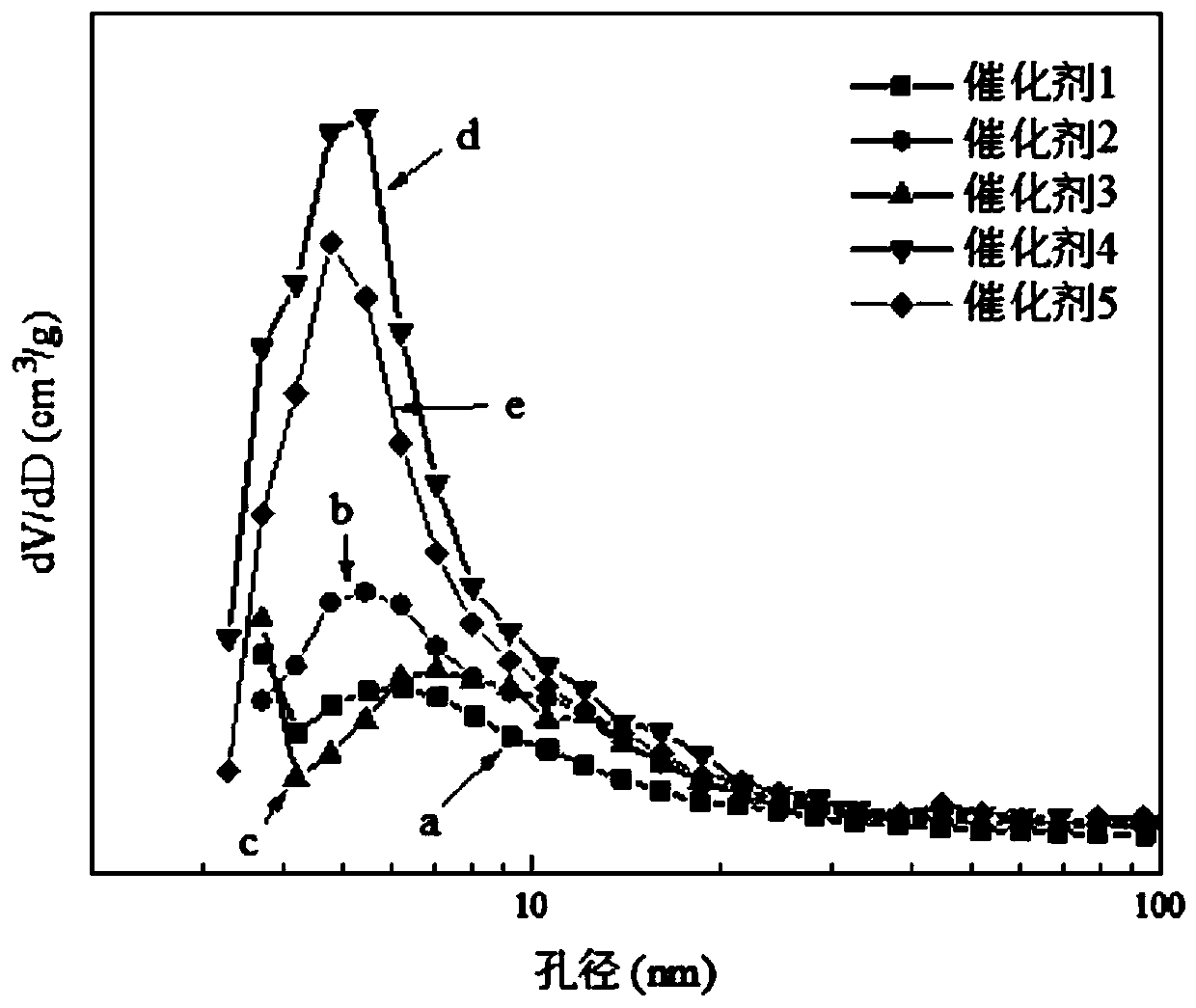
[0028] (1) Mix 1.2 g of MOR molecular sieve and 0.3 g of β molecular sieve into a composite molecular sieve carrier, and stir for 5 minutes. (The content of MOR molecular sieve and β molecular sieve in the composite molecular sieve carrier is 80% and 20%, respectively; the specific surface area, pore volume and most probable pore size of the composite molecular sieve are 302.5 m, respectively 2 / g, 0.25cm 3 / g and 6.3nm)
[0029] (2) 0.06g of (NH 4 ) 6 Mo 7 O 24 ·4H 2 O and 0.03 g of Co(NO 3 ) 2 ·6H 2O and 0.03 g of Ce (NO 3 ) 3 ·6H 2 O was dissolved in 20 mL of deionized water, and a highly dispersed solution was obtained after stirring for 0.5 h.
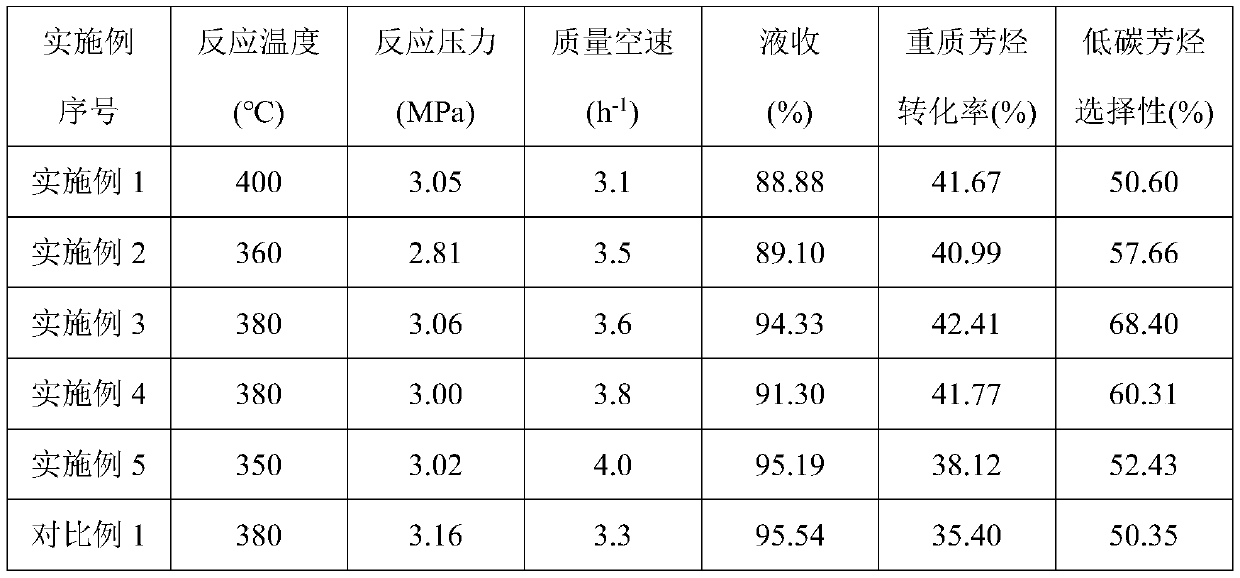
[0030] (3) The molecular sieve obtained in step (1) was immersed in the precursor mixed solution obtained in step (2) for 24 hours, dried at 110° C. for 8 hours, and calcined at 400° C. for 4 hours in an air atmosphere to obtain a finished catalyst 1. (MoO in the catalyst 3 The content is 3%, Co 3 O 4 Content is 0....
Embodiment 2
[0032] (1) Mix 0.9 g of MOR molecular sieve, 0.3 g of β molecular sieve and 0.3 g of ZSM-5 molecular sieve into a composite molecular sieve carrier, and stir for 6 minutes. (The content of MOR molecular sieve, β molecular sieve and ZSM-5 molecular sieve in the composite molecular sieve carrier is 60%, 20% and 20%, respectively; the specific surface area, pore volume and most probable pore size of the composite molecular sieve are 321.2 m 2 / g, 0.35cm 3 / g and 5.4nm)
[0033] (2) 0.12 g of (NH 4 ) 6 Mo 7 O 24 ·4H 2 O and 0.12 g of Ni (NO 3 ) 2 ·6H 2 O and 0.005g of La (NO 3 ) 3 ·6H 2 O was dissolved in 20 mL of deionized water, and a highly dispersed solution was obtained after stirring for 1 h.
[0034] (3) The molecular sieve obtained in step (1) was immersed in the precursor mixed solution obtained in step (2) for 30 hours, dried at 120° C. for 10 hours, and calcined at 450° C. for 6 hours in an air atmosphere to obtain finished catalyst 2. (MoO in the catalyst ...
Embodiment 3
[0036] (1) Mix 0.9 g of MOR molecular sieve, 0.3 g of Y molecular sieve and 0.3 g of ZSM-5 molecular sieve into a composite molecular sieve carrier, and stir for 7 minutes. (The content of MOR molecular sieve, Y molecular sieve and ZSM-5 molecular sieve in the composite molecular sieve carrier is 60%, 20% and 20%, respectively; the specific surface area, pore volume and most probable pore size of the composite molecular sieve are 385.6 m 2 / g, 0.37cm 3 / g and 7.0nm)
[0037] (2) 0.18 g of (NH 4 ) 6 Mo 7 O 24 ·4H 2 O and 0.045g of Bi (NO 3 ) 3 ·5H 2 O and 0.015g of La (NO 3 ) 3 ·6H 2 O was dissolved in 20 mL of deionized water, and a highly dispersed solution was obtained after stirring for 1.5 h.
[0038] (3) The molecular sieve obtained in step (1) was immersed in the precursor mixed solution obtained in step (2) for 36 hours, dried at 130° C. for 12 hours, and calcined at 500° C. for 6 hours in an air atmosphere to obtain finished catalyst 3. (MoO in the catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com