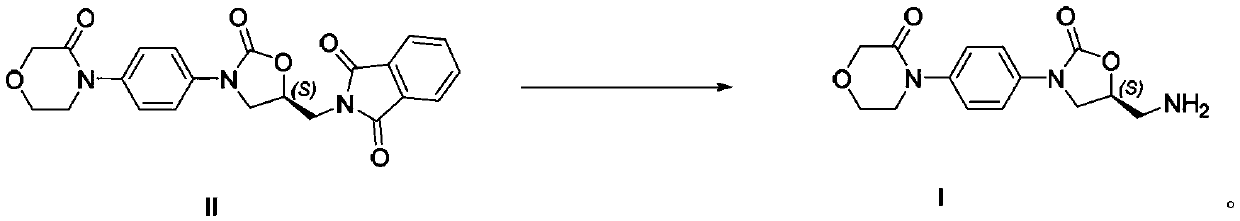
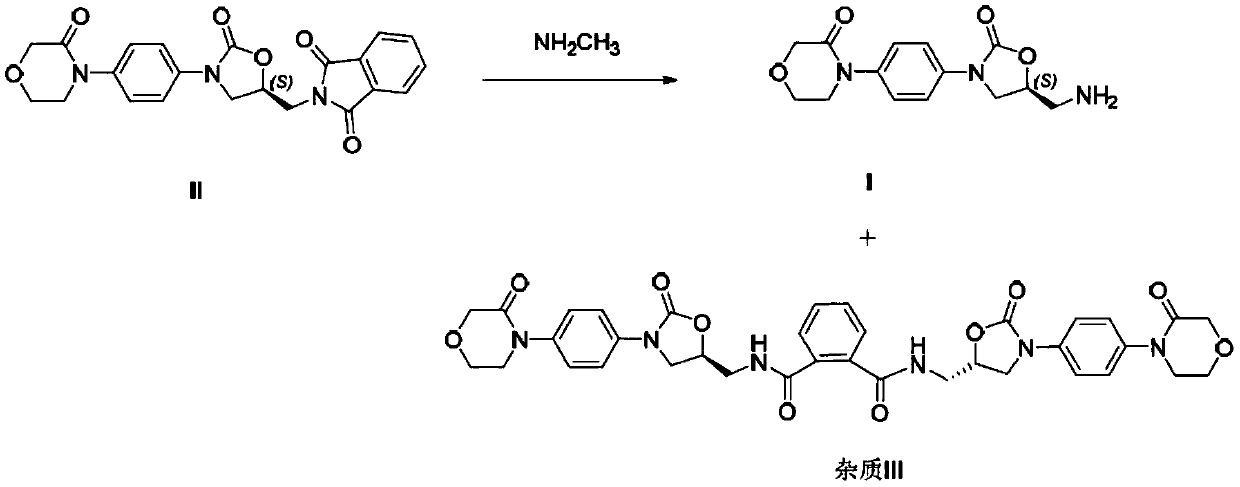
Rivaroxaban intermediate preparation method
A compound and organic solvent technology, applied in the field of drug synthesis, can solve problems such as high requirements for production equipment, difficult removal, environmental pollution, etc., and achieve the effects of avoiding the use of high-temperature and high-pressure equipment, improving process safety, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Put the compound of formula II (42.1g), ethanol (252mL) and n-butylamine (47.2g, 8eq) into the reaction flask, heat up to 75-80°C, and keep the reaction for 8-12 hours. After the reaction, the reaction solution was cooled to Stir at 0-5°C for 1 hour, then filter the obtained solid, rinse the filter cake with ethanol (20 mL) and dry to obtain the intermediate compound of formula I (23.3 g), with a purity (HPLC) of 99.57% and a yield of 80%.
[0031] The characterization data of the intermediate formula I compound prepared in Example 1 are as follows: mp: 149-151° C.; [α] 20 D (C=0.01g / mL, water)=-59.4°; TOF-MS (ES + ): 292.16[M+H]; 1 H NMR (DMSO-d 6 , 400MHz) δ: 7.56(d, J=8.8Hz, 2H), 7.41(d, J=8.8Hz, 2H), 4.93~4.88(m, 2H), 4.18(s, 2H), 4.15(m, 1H ), 4.16(d, J=8.8Hz, 1H), 3.98~3.92(m, 3H), 3.71(t, J=5.2Hz, 2H), 3.14~3.10(m, 2H); 13 C NMR (DMSO-d 6 , 100MHz) δ: 166.16, 153.98, 137.34, 136.57, 126.03, 118.70, 70.84, 67.87, 63.63, 49.17, 47.45, 42.32.
Embodiment 2 to Embodiment 9
[0033] According to the operation process of embodiment 1, under the situation that the charging capacity (42.1g) of compound of formula II remains constant, change solvent and consumption thereof, the consumption of n-butylamine and reaction temperature, the results are shown in the following table 1.
[0034] Table 1
[0035] Example number Solvent and dosage The dosage of n-butylamine temperature reflex yield 2 Ethanol (252mL) 64.9g (11eq) 75~80℃ 88% 3 Ethanol (252mL) 88.5g (15eq) 75~80℃ 86% 4 Ethanol (421mL) 64.9g (11eq) 75~80℃ 84% 5 Methanol (252mL) 64.9g (11eq) 60~65℃ 78% 6 n-propanol (252mL) 64.9g (11eq) 75~80℃ 83% 7 Isopropanol (252mL) 64.9g (11eq) 75~80℃ 82% 8 n-Butanol (252mL) 64.9g (11eq) 95~100℃ 85% 9 tert-butanol (252mL) 64.9g (11eq) 75~80℃ 81%
[0036] As can be seen from Table 1, embodiment 2 is optimum process condition.
[0037] After testing, the characterizat...
Embodiment 10
[0038] Embodiment 10: under above-mentioned optimal process condition, replace n-butylamine with n-propylamine
[0039] Put the compound of formula II (42.1g), ethanol (252mL), and n-propylamine (64.9g, 11eq) into the reaction flask, raise the temperature to 75-80°C, and keep the reaction for 20-24 hours. After the reaction, cool the reaction solution to 0 ~5°C, heat preservation and stirring for 1 hour, filter the resulting solid, rinse the filter cake with ethanol (10 mL) and dry to obtain the intermediate compound of formula I (23.9 g), with a purity (HPLC) of 99.8% and a yield of 82%.
[0040] After testing, the characterization data of the intermediate formula I compound prepared in Example 10 is consistent with the characterization data of the intermediate formula I compound prepared in Example 1.
[0041] The above embodiments are only a part of the preferred implementation of the present invention, and from a technical perspective, some optimizations in the described i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com