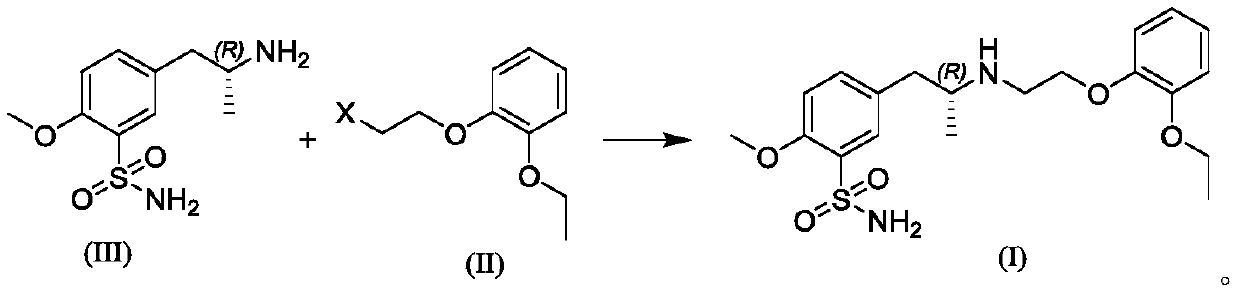
Preparation method of tamsulosin hydrochloride
A technology of tamsulosin hydrochloride and hydrochloride, applied in the field of preparation of tamsulosin hydrochloride, can solve the problems of high reaction cost, environmental pollution, optimization, etc., and achieves avoiding flammable gas, improving process safety, reducing The effect of process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
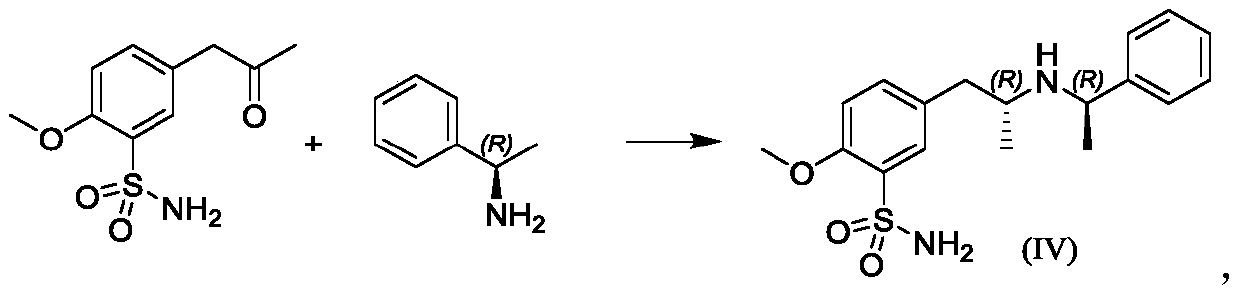
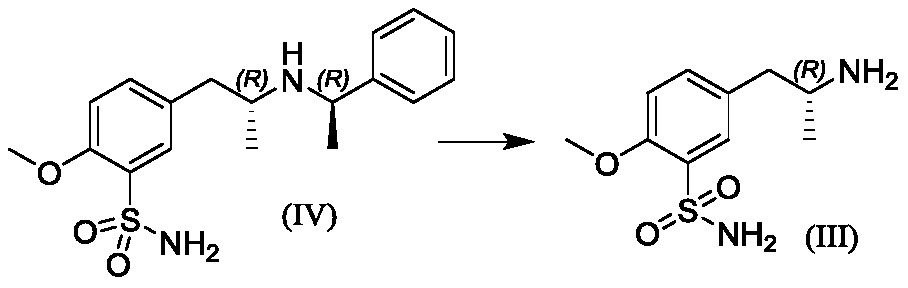
[0043] Step 1, Synthesis of 5-[[(2R)-2-[(1R)-N-(1-methylbenzyl)]amino]propyl]-2-methoxysulfonamide hydrochloride
[0044] Put dichloromethane (2250mL, 15V) and (R)-1-phenylethylamine (150.0g, 1.0eq) into a 10L three-necked flask, start stirring, and add 5-acetonyl-2-methoxybenzenesulfonamide (316.2g, 1.05eq), slowly add acetic acid (74.3g, 1.0eq), warm up to 30-35°C, add sodium triacetoxyborohydride (314.8g, 1.2eq), stir for 16h, stop the reaction. Add dropwise 3mol / L dilute hydrochloric acid aqueous solution (958.0g), stir for 30min, add dropwise 30% sodium hydroxide aqueous solution (1096.0g), stir and separate layers, add saturated saline (1500.0g) to the organic phase, stir for 15min, pass diatom Soil (30.0g), separate the organic phase, add 33% hydrogen chloride ethanol solution (225.0g) dropwise, stir for 60 minutes, evaporate a large amount of solvent under reduced pressure, add acetonitrile (2250mL), stir for 30min, filter, and wash the wet product with acetonitrile ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com