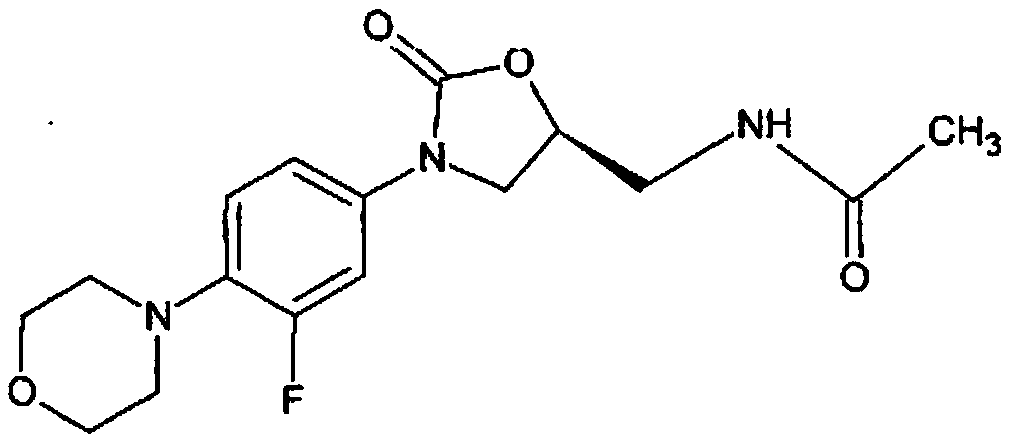
Method for testing particle size and particle size distribution of linezolid raw medicine
A technology of linezolid and a testing method, which is applied in the field of testing the particle size and particle size distribution of linezolid bulk drugs, can solve the problems of difficulty in preparing samples, unsuitability, easy agglomeration and the like, and achieves simple, convenient and good reproducibility in the determination process. Effects of Sex and Precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
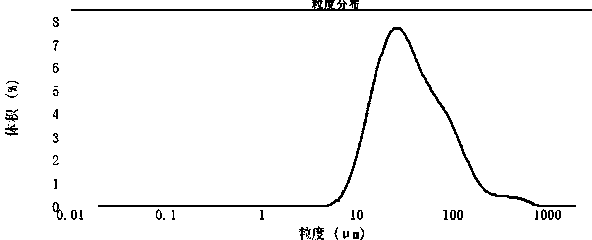
[0027] Example 1 The particle size determination of linezolid bulk drug under different shading degrees
[0028] (1) Preparation before experiment:
[0029] Use the Malvern laser particle size analyzer, turn on the instrument, and warm up for half an hour. Run the particle size testing software, use the saturated solution of linezolid API to clean the pipeline of the instrument, and manually test the link in the software window to test the background of the instrument.
[0030] (2) Preparation of sample suspension: Take about 0.3g of linezolid API sample in a 100mL beaker, add 0.2ml of surfactant Tween-80 aqueous solution (W / V: 0.54g / 100ml) for wetting , and then add 50ml of saturated aqueous solution of linezolid bulk drug, and fully stir until a homogeneous suspension is obtained.
[0031] (3) Instrument parameter setting:
[0032] Injector: manual wet sampler with small capacity;
[0033] Sample refractive index: 1.60;
[0034] Sample absorbance: 0;
[0035] Dispersio...
Embodiment 2
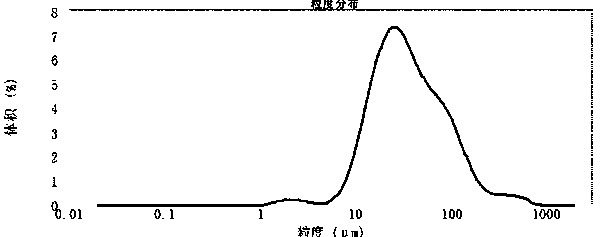
[0045] Embodiment 2 intermediate precision experiment
[0046] The same batch of crude drug is carried out particle size distribution test by the method of the present invention by two analysts on the next day. The first analyst took 6 samples, each sample was tested 6 times in parallel, and the average and RSD of d(0.1), d(0.5), d(0.9) were counted; the second analyst performed the same batch of API samples For re-examination, 6 samples were taken in parallel and tested 6 times for each sample, and the average value and RSD of d(0.1), d(0.5), and d(0.9) were counted. Calculate the mean and RSD of d(0.1), d(0.5), d(0.9) for 6 samples each for two analysts, and the mean of d(0.1), d(0.5), d(0.9) for a total of 12 samples Value and RSD.
[0047] (1) Preparation before experiment:
[0048] Use the Malvern laser particle size analyzer, turn on the instrument, and warm up for half an hour. Run the particle size testing software, use the saturated aqueous solution of linezolid A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com