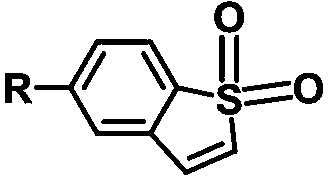
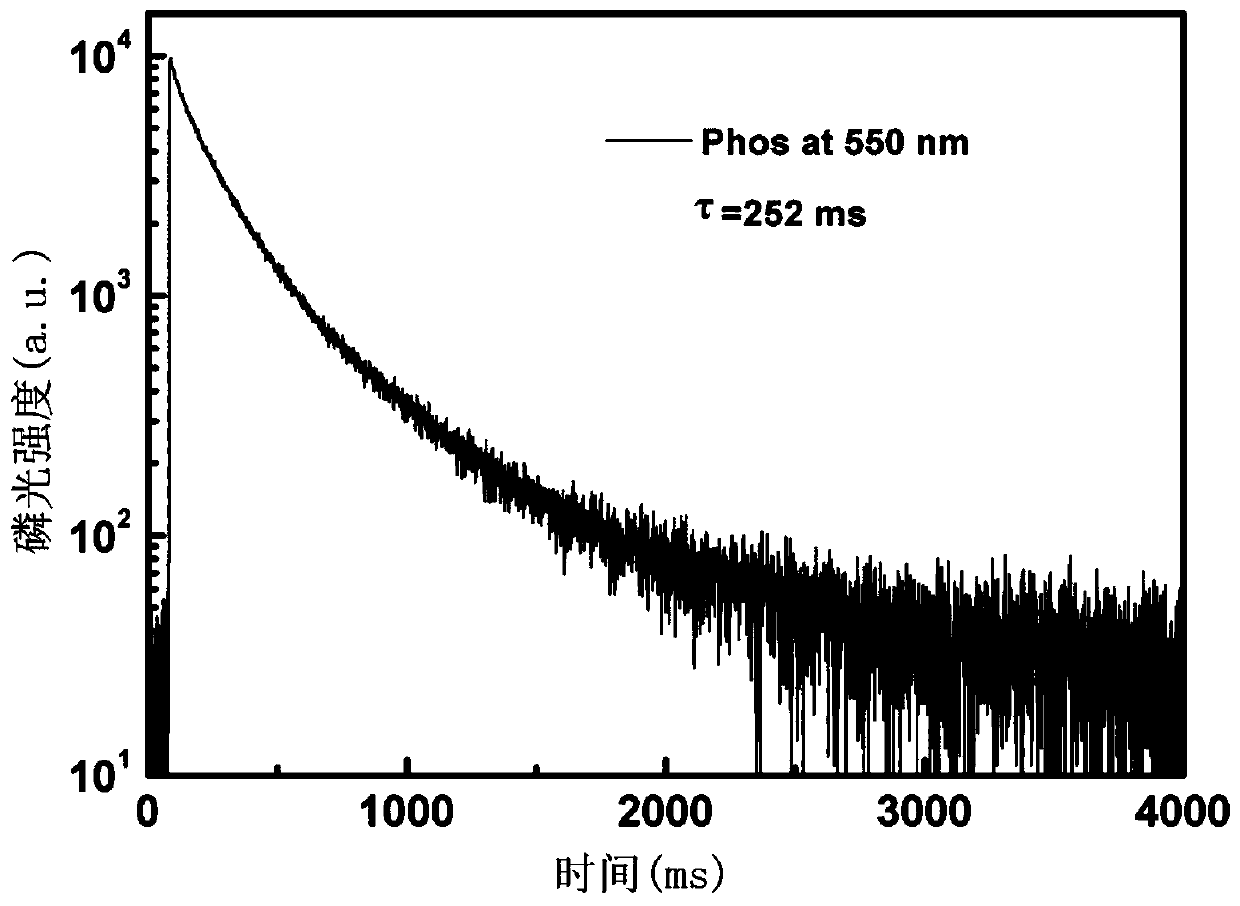
Benzothiophene derivative room-temperature phosphorescent material and preparation method thereof
A room-temperature phosphorescence and benzothiophene technology, which is applied in the fields of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as low lifespan, limited practical application, and few types of room-temperature phosphorescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In this example, the benzothiophene derivative room temperature phosphorescent material was prepared according to the following reaction formula:
[0026]
[0027] Specifically: 5-bromobenzo[b]thiophene 1,1-dioxide (2.43g, 10.0mmol), phenoxazine (2.07g, 11.0mmol), Pd(OAc) 2 (0.0225g, 0.1mmol), t-BuONa (0.0192g, 0.2mmol) and P( t Bu) 3 (0.1mL) was added into a round-bottomed flask containing 100mL toluene, argon was blown for 30min, and then under argon protection, the reaction was carried out at 110°C for 72h. 100 mL) was extracted three times, and the organic phases were combined and dried over anhydrous sodium sulfate. The solvent was removed to obtain a crude product, which was then purified by column chromatography to obtain the product (3.1 g, 89.2%).
[0028] The H NMR spectrum data are as follows: 1 HNMR (500MHz, CDCl 3 )δ=7.88(d,J=7.5,1H),7.46(d,J=1.4,1H),7.38–7.27(m,2H),7.23–7.01(m,8H),6.67(d,J=10.8 ,1H).MS:(MALDI-TOF)m / z calcd for C 20 h 13 NO 3 S,...
Embodiment 2
[0030] In this example, the benzothiophene derivative room temperature phosphorescent material was prepared according to the following reaction formula:
[0031]
[0032] Specifically: 5-bromobenzo[b]thiophene 1,1-dioxide (2.43g, 10.0mmol), carbazole (1.83g, 11.0mmol), Pd(OAc) 2 (0.0225g, 0.1mmol), t-BuONa (0.0192g, 0.2mmol) and P( t Bu) 3 (0.1mL) was added into a round-bottomed flask containing 100mL toluene, argon was blown for 30min, and then under argon protection, the reaction was carried out at 110°C for 72h. 100 mL) was extracted three times, and the organic phases were combined and dried over anhydrous sodium sulfate. The solvent was removed to obtain a crude product, which was then purified by column chromatography to obtain the product (2.8 g, 84.5%).
[0033] The H NMR spectrum data are as follows: 1 H NMR (500MHz, Chloroform) δ=8.11–7.95(m,4H),7.62(dd,J=7.5,1.6Hz,1H),10.41–5.94(m,13H),8.28–5.94(m,12H), 7.48–7.18 (m, 6H), 6.67 (d, J=10.8Hz, 1H). MS: (MALDI-...
Embodiment 3
[0035] In this example, the benzothiophene derivative room temperature phosphorescent material was prepared according to the following reaction formula:
[0036]
[0037] Specifically: 5-bromobenzo[b]thiophene 1,1-dioxide (2.43g, 10.0mmol), carbazole (2.29g, 11.0mmol), Pd(OAc) 2 (0.0225g, 0.1mmol), t-BuONa (0.0192g, 0.2mmol) and P( t Bu) 3 (0.1mL) was added to a round-bottomed flask containing 100mL of toluene, argon was blown for 30min, and then under argon protection, the reaction was carried out at 110°C for 72h. After cooling to room temperature, the solvent was removed by evaporation, and then extracted with chloroform (100mL) Three times, the organic phases were combined and dried over anhydrous sodium sulfate. The solvent was removed to obtain a crude product, which was then purified by column chromatography to obtain the product (3.2 g, 85.7%).
[0038] The H NMR spectrum data are as follows: 1 H NMR (500MHz, CDCl 3 )δ=7.87(s,1H),7.63(s,1H),7.51(s,1H),7.33(s,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com