Medical intermediate compound and synthesis method thereof
A synthesis method and compound technology, applied in the fields of drug combination, organic chemistry, bulk chemical production, etc., can solve the problems of catechol group without method, not method, improvement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
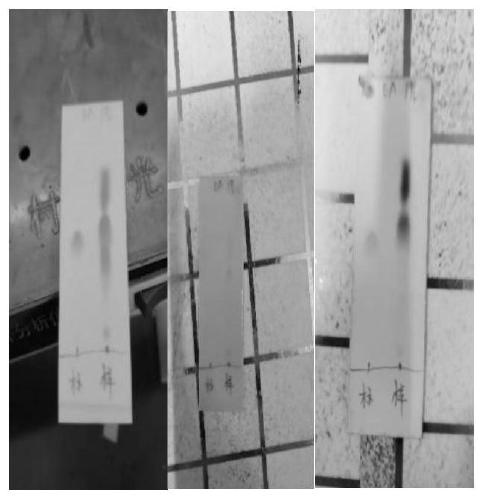
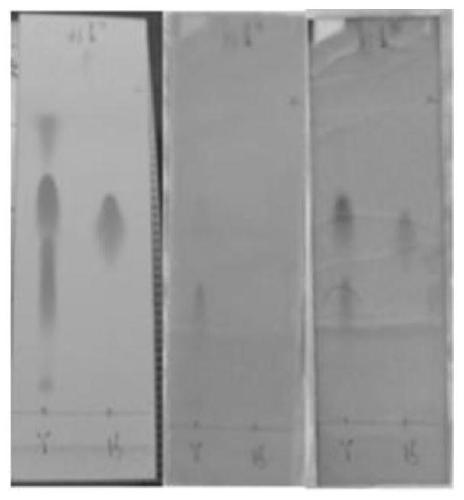
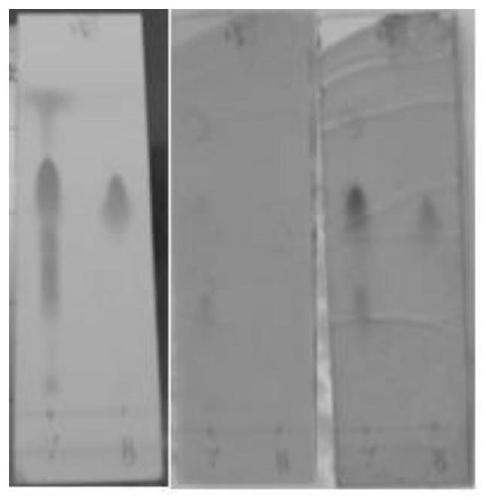
Image
Examples
Embodiment 1
[0066] Embodiment 1: the impact of strong acid TsOH catalysis on the reaction of acetone
[0067] (1) Add sodium tetraborate decahydrate (19.1g, 50mmol) and water (250ml) into a 500ml three-neck flask, stir with a magnetic stirrer, and pass through argon to deoxygenate for 30 minutes. Then add L-DOPA (19.7g, 100mmol) and Na to the mixed solution 2 CO 3 (10.6g, 100mmol), get Fmoc-OSu (33.8g, 100mmol), be dissolved in 200ml THF, adopt dropping funnel to add reaction body dropwise, mixture is stirred at room temperature 4 hours, adopt thin-layer chromatography (TLC) to monitor Response progress. Use 1N HCl solution to acidify the pH value of the reaction system to pH = 1, rotary evaporate THF in the mixed solution, use EtOAc to extract the mixed solution, take the organic layer, and wash with MgSO 4 After drying to remove water, the product was dried in a vacuum oven after rotary evaporation to obtain Fmoc-DOPA-OH as a foamy white solid (37 g, 88%).
[0068] (2) Add Fmoc-DOPA...
Embodiment 2
[0069] Embodiment 2: a kind of synthetic method of Fmoc-DOPA (Acetonide)-OH, specifically comprises the following steps:
[0070] (1) Add sodium tetraborate decahydrate (19.1g, 50mmol) and water (250ml) into a 500ml three-neck flask, stir with a magnetic stirrer, and pass through argon to deoxygenate for 30 minutes. Then add L-DOPA (19.7g, 100mmol) and Na to the mixed solution 2 CO 3 (10.6g, 100mmol), get Fmoc-OSu (40.48g, 120mmol), be dissolved in 200ml THF, adopt dropping funnel to add reaction body dropwise, mixture is stirred at room temperature 4 hours, adopt thin-layer chromatography (TLC) to monitor Response progress. Use 1N HCl solution to acidify the pH value of the reaction system to pH = 1, rotary evaporate THF in the mixed solution, use EtOAc to extract the mixed solution, take the organic layer, and wash with MgSO 4 After drying to remove water, the product was dried in a vacuum oven after rotary evaporation to obtain Fmoc-DOPA-OH as a foamy white solid (39.9 g...
Embodiment 3
[0072] Embodiment 3 A kind of synthetic method of Fmoc-DOPA (Acetonide)-OH, specifically comprises the following steps:
[0073] (1) Add sodium tetraborate decahydrate (19.1g) and water (250ml) into a 500ml three-necked flask, stir with a magnetic stirrer, feed argon to remove oxygen for 30 minutes, then add L- DOPA (19.7g) and Na 2 CO 3 (5.6g), get Fmoc-OSu (40.48g), be dissolved in 50ml THF, adopt drop funnel to add reaction body dropwise, mixture is stirred at room temperature 4 hours, adopt thin-layer chromatography (TLC) to monitor reaction progress situation. Use 1N HCl solution to acidify the pH value of the reaction system to pH = 1, rotary evaporate THF in the mixed solution, use EtOAc to extract the mixed solution, take the organic layer, and wash with MgSO 4 After drying to remove water, the product was dried in a vacuum oven after rotary evaporation to obtain Fmoc-DOPA-OH as a foamy white solid (37.5 g, 89%).
[0074] (2) Take the dried Fmoc-DOPA-OH (2.1g) inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com