Application of bacillus subtilis fibrinolytic enzyme in treating bronchitis
A technology of Bacillus subtilis and bronchitis, applied in the application field of Bacillus subtilis fibrinolytic enzyme in the treatment of bronchitis drugs, can solve the problems of treating symptoms but not the root cause, chronic bronchitis cannot be cured, lack of chronic bronchitis treatment drugs, etc., to achieve cough relief good effect, good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
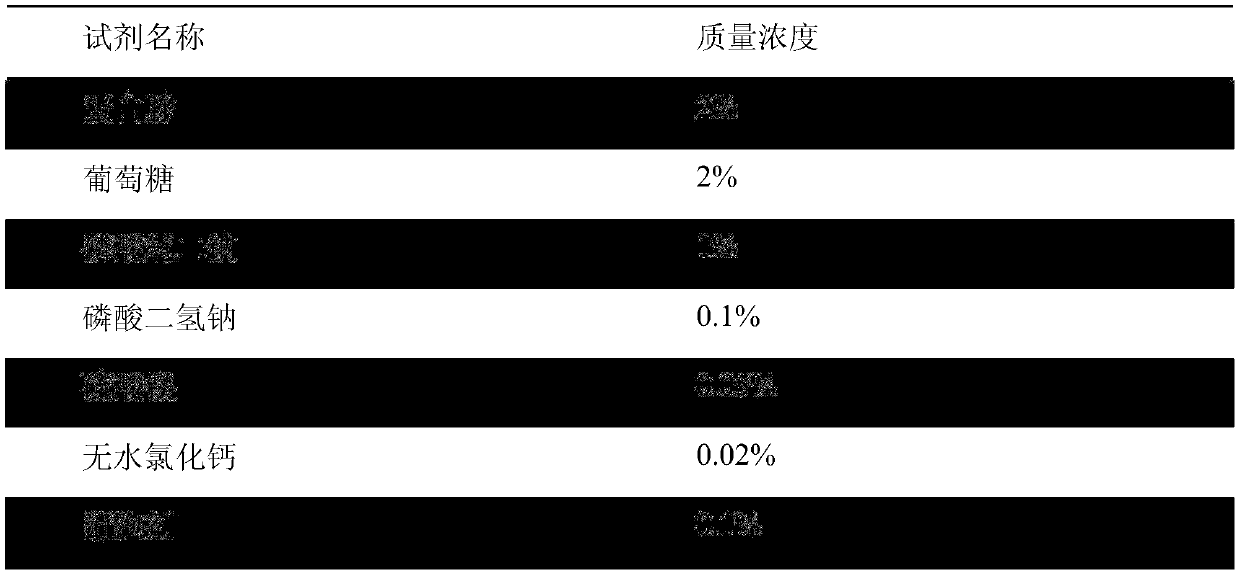
[0016] 3. Preparation of Bacillus subtilis fibrinolytic enzyme powder
[0017] After the fermentation is finished, the fermented liquid is centrifuged by a tube centrifuge, concentrated by microfiltration and ultrafiltration to obtain a concentrated liquid of about 25 L. The concentrated solution is spray-dried to obtain the spray-dried powder of subtilis stalk fibrinolytic enzyme raw material. The spray drying condition is that the inlet air temperature is 140°C and the outlet air temperature is 63-68°C;
[0018] 2. Implementation
Embodiment 1
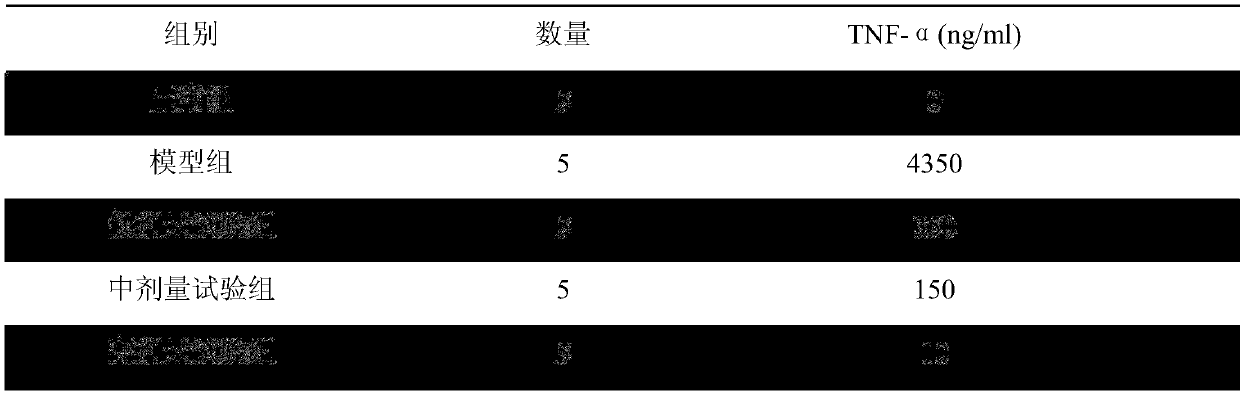
[0020] 1. Animals used in the test: 25 rats, weighing 30-35g, were randomly divided into 5 groups, marked as normal group, model group, low-dose test group, middle-dose test group, and high-dose test group;
[0021] 2. Sample preparation
[0022] Normal group: normal saline, once a day, 0.3ml each time;
[0023] Blank control group: normal saline, once a day, 0.3ml each time;
[0024] Low-dose test group: 500 IU subtilis fibrinolytic enzyme solution was prepared with physiological saline, once a day, 0.3ml each time, for 30 consecutive days;
[0025] Medium-dose test group: prepare 500 IU subtilis fibrinolytic enzyme solution with physiological saline, once a day, 0.3ml each time, for 30 consecutive days;
[0026] High-dose test group: 500 IU subtilis fibrinolytic enzyme solution was prepared with physiological saline, once a day, 0.3ml each time, for 30 consecutive days;
[0027] 3. Test method
[0028] The rats in the model group and the three test groups were all treate...
Embodiment 2
[0039] 1. Animals used in the test: 25 rats, weighing 30-35g, were randomly divided into 5 groups, marked as normal group, model group, low-dose test group, middle-dose test group, and high-dose test group;
[0040] 2. Sample preparation
[0041] Blank control group: normal saline;
[0042] Positive control group: dextromethorphan hydrobromide tablets (manufactured by Guangzhou Baiyunshan Guanghua Pharmaceutical Co., Ltd., batch number H10900005), which were dissolved in normal saline to make a suspension with a concentration of 3 mg / ml and a dose of 35 mg / kg weight;
[0043] Low-dose test group: prepare subtilis plasmin solution with a concentration of 500IU with physiological saline, and the dose is 35mg / kg body weight / day;
[0044] Medium-dose test group: the subtilis plasmin solution with a concentration of 500 IU was prepared with physiological saline, and the dose was 35 mg / kg body weight / day;
[0045] High-dose test group: prepare 500 IU subtilis fibrinolytic enzyme ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com