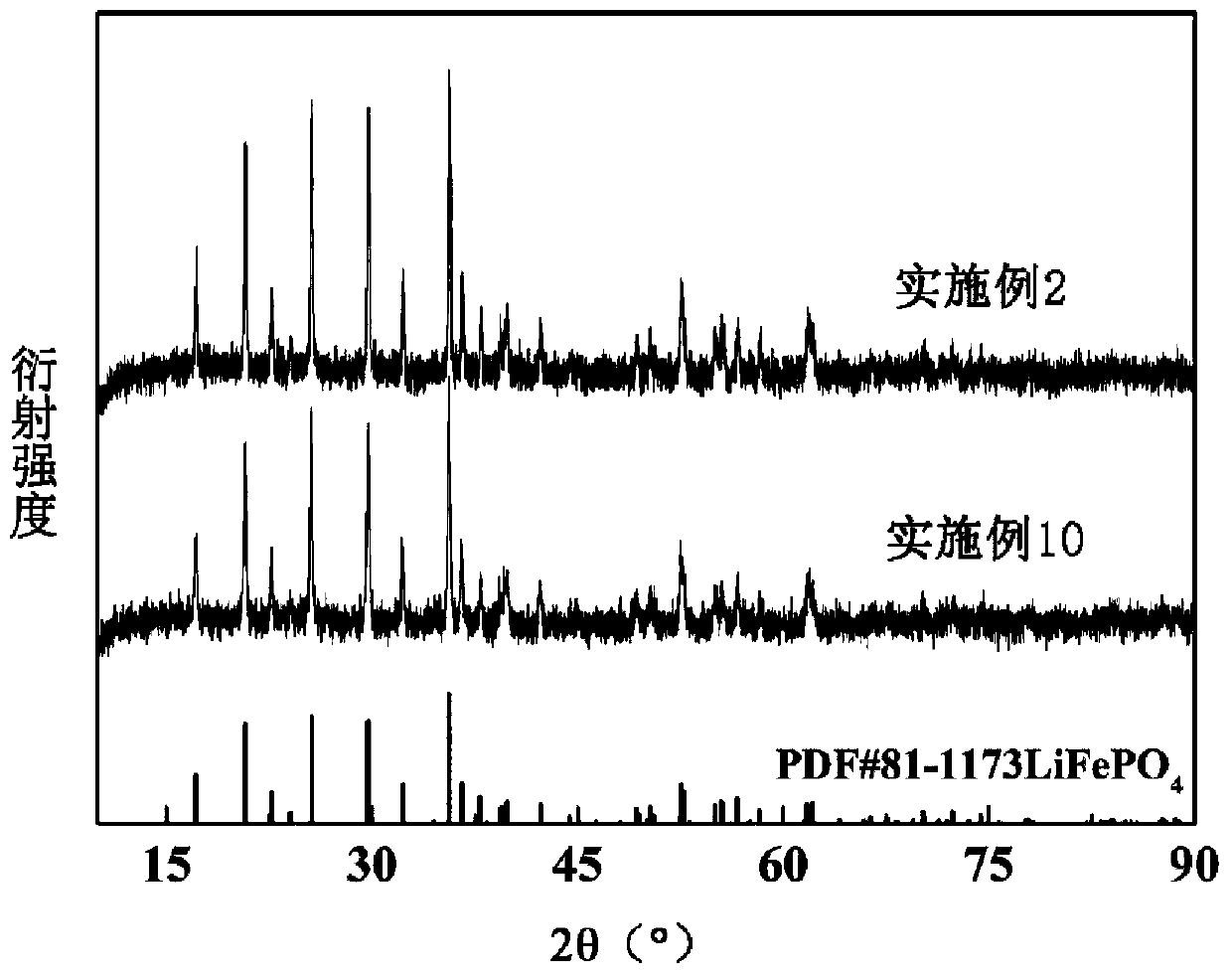
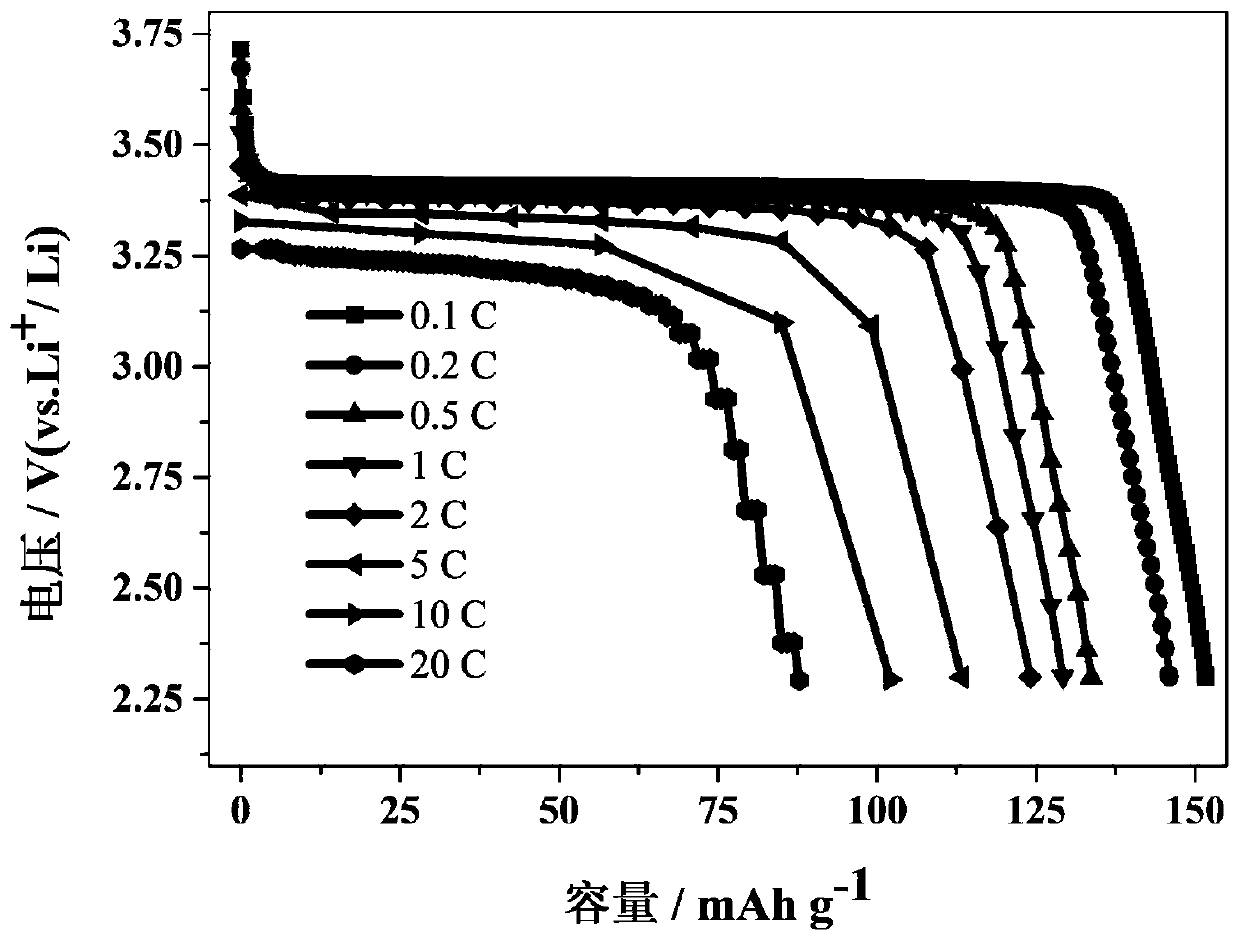
Method for preparing lithium iron phosphate positive electrode material by taking pickling iron oxide red as raw material
A cathode material, a technology for lithium iron phosphate, which is applied in the field of preparation of lithium iron phosphate cathode materials, can solve the problems of unsuitable large-scale production, cumbersome processing of iron red, etc., to alleviate the energy and environmental crisis, high rate performance, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] In this example, the lithium iron phosphate positive electrode material was prepared using pickled iron red as a raw material according to the following method:
[0079] (1) Accurately weigh pickling iron red 500g, battery grade lithium carbonate 237.5g, and battery grade ammonium dihydrogen phosphate 718.8g, citric acid 401.3g and aluminum isopropoxide 50g, add deionized water in the stirring mill, Add the raw materials of the above quality into the ball mill for 4 hours in order, add an appropriate amount of deionized water to the mixed material, and adjust the solid-liquid ratio of the uniform slurry to 95%. Subsequently, the slurry was dried in an oven at 120° C. for 24 h.
[0080] (2) Under a nitrogen atmosphere, raise the temperature from room temperature to 350°C at a heating rate of 5°C / min, keep the temperature for 8 hours, cool to room temperature and pulverize, then raise the heating rate to 750°C at a heating rate of 5°C / min, and keep it for 10 hours. After...
Embodiment 2
[0084] In this example, the lithium iron phosphate positive electrode material was prepared using pickled iron red as a raw material according to the following method:
[0085] (1) Accurately weigh pickling iron red 500g, battery grade lithium carbonate 237.5g, and battery grade ammonium dihydrogen phosphate 718.8g, citric acid 401.3g and ammonium chloride 18.8g respectively, add deionized water in the stirring mill, Add the raw materials of the above quality and ball mill for 4 hours in sequence, add an appropriate amount of deionized water to the mixed material, and adjust the solid-to-liquid ratio of the homogeneous slurry to 25%. Subsequently, the slurry was placed in an oven and dried at 120° C. for 24 hours to obtain a mixture.
[0086] (2) Under a nitrogen atmosphere, the mixture prepared in step (1) was raised from room temperature to 350°C at a heating rate of 5°C / min, kept for 8 hours, cooled to room temperature, and pulverized, and then heated again at a heating rat...
Embodiment 3
[0093] In this example, the lithium iron phosphate positive electrode material was prepared using pickled iron red as a raw material according to the following method:
[0094] (1) Accurately weigh pickling iron red 500g, battery grade lithium carbonate 237.5g, and battery grade ammonium dihydrogen phosphate 718.8g, citric acid 401.3g and sodium carbonate 18.8g respectively, add deionized water in the stirring mill, according to Sequentially add the raw materials of the above quality and ball mill for 4 hours, add an appropriate amount of deionized water to the mixed material, and adjust the solid-to-liquid ratio of the homogeneous slurry to 25%. Subsequently, the slurry was dried in an oven at 120° C. for 24 h.
[0095] (2) Under an argon atmosphere, raise the temperature from room temperature to 350°C at a rate of 5°C / min, keep it warm for 8 hours, cool to room temperature and pulverize it, then raise it to 750°C at a rate of 5°C / min, and keep it warm for 10 hours , after c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com