A dual-mode separation immunosensor based on enzyme-induced bioetching and its preparation method
An immune sensor and separate technology, applied in the field of immune sensors, can solve the problems of long operation time, inaccurate detection results, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method based on enzyme-induced bioetching dual-mode separation immunosensor, comprising the following steps:
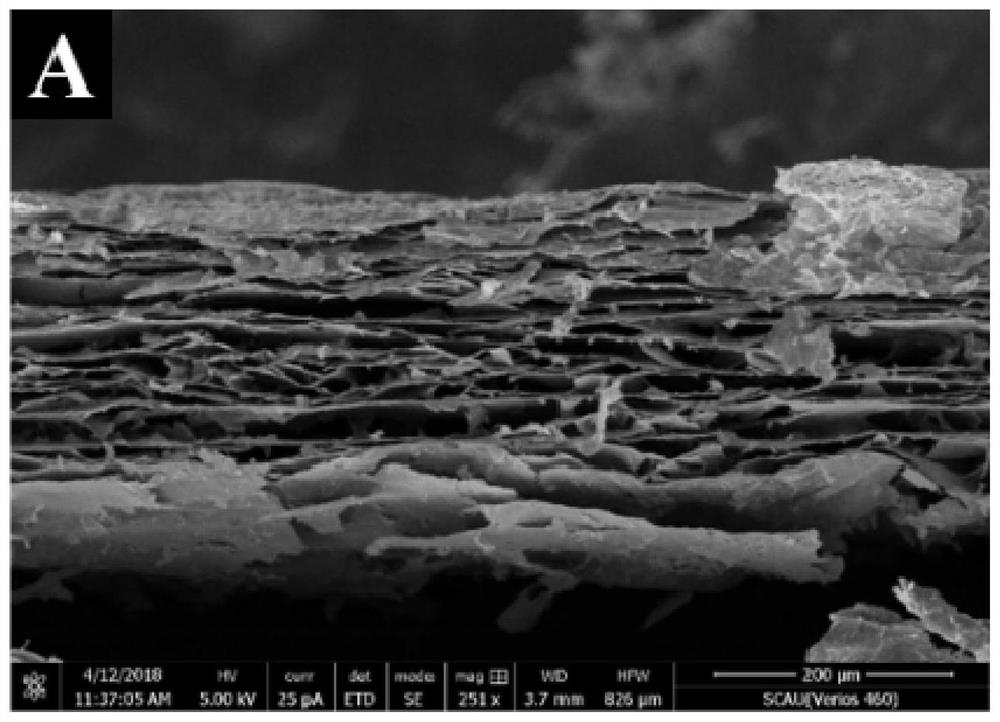
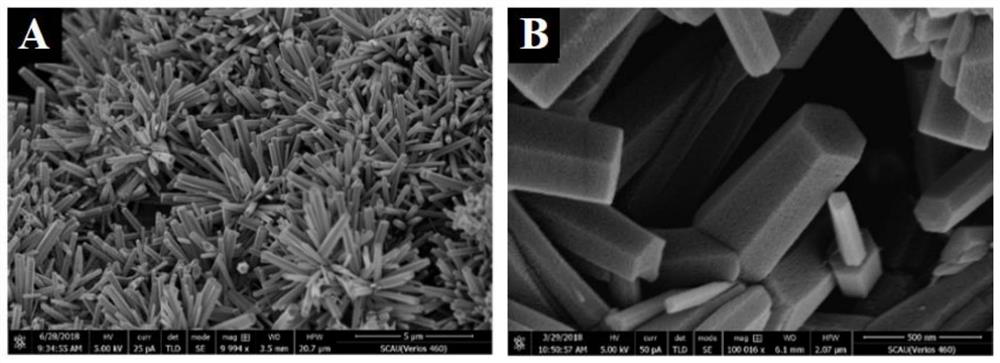
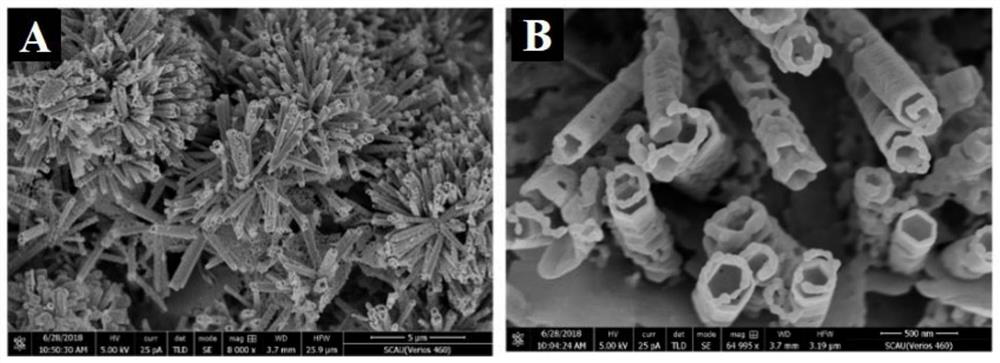
[0036] S1. Preparation of CdS / ZnO NRs / r-GO: first synthesize graphene oxide hydrogel (GO), and prepare cadmium sulfide / zinc oxide nanorod array CdS / ZnO NRs on the reduced graphene oxide r-GO to obtain CdS / ZnO NRs / r-GO composites;
[0037] Preparation of HRP-liposome-Ab 2 Complex: preparation of liposome-encapsulated horseradish peroxidase HRP by thin-film dispersion method,
[0038] The HRP-liposome complex was obtained, and the HRP-liposome complex was combined with the second antibody Ab by glutaraldehyde cross-linking method 2 connect,
[0039] HRP-Liposome-Ab 2 Complex;
[0040] Preparation of gold nanobicones: Au NBPs prepared by seed growth method;
[0041] The specific operation for preparing CdS / ZnO NRs / r-GO is as follows: add 160mL concentrated sulfuric acid into a dry three-necked flask, slowly add 4g graphite powder and 14g potas...
Embodiment 2
[0050] Example 2 Characterization of CdS / ZnO NRs / r-GO composites
[0051] Synthesis of graphene oxide hydrogel (GO): Add 160mL concentrated sulfuric acid into a dry three-necked flask, slowly add 4g graphite powder and 14g potassium permanganate under stirring in an ice-water bath, and keep stirring the mixture at 35°C for 24h . After the reaction was completed, dilute with 400 mL of ice water, and then add hydrogen peroxide until the color of the mixture did not change and no bubbles were generated. Stirring was continued for 2 h to remove unreacted potassium permanganate, and then centrifuged at 5000 rpm for 3 minutes. Centrifuge and wash with 300mL HCl (1mol / L) three times and then wash with water until the supernatant is neutral. The precipitate after centrifugation was dialyzed for one week, and the product was divided into two equal parts, which were centrifuged and washed with water and ethanol respectively, and finally dispersed in water or ethanol and stored for late...
Embodiment 3
[0054] Example 3 HRP-liposome-Ab 2 Characterization of the complex
[0055] The liposome-encapsulated horseradish peroxidase (HRP) complex is prepared by a film dispersion method, and the process is briefly described as follows: Weigh dipalmitoylphosphatidylcholine (DPPC), cholesterol and dipalmitoylphosphatidylethanolamine (DPPE ) in a total of 30 mg (molar ratio 10:10:1), mixed and dissolved in 4 mL of chloroform / methanol mixture (volume ratio 6:1), and then transferred to a round-bottomed flask by rotary evaporation at 45 ° C to form a uniform layer Lipid film, then add 2 mL of phosphate buffer solution (PBS, 0.01 M, pH 7.4) containing 2.5 mg / mL HRP, and incubate at 35 °C for 2 h. The mixture was then sonicated for 5 min in an ice-water bath, repeating three cycles. Finally, the prepared HRP-liposome complex was centrifuged to remove unencapsulated HRP.
[0056] The complexes prepared above were combined with the second antibody (Ab) by glutaraldehyde cross-linking metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com