Recombinant bacillus subtilis strain expressing and secreting beta-mannanase efficiently and method for obtaining strain
A technology of Bacillus subtilis and mannanase, applied in the field of molecular biology, can solve the problem of predicting the most effective signal peptide sequence without downstream protein sequence, affecting the correct folding of β-mannanase, and β-mannanase Degradation and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
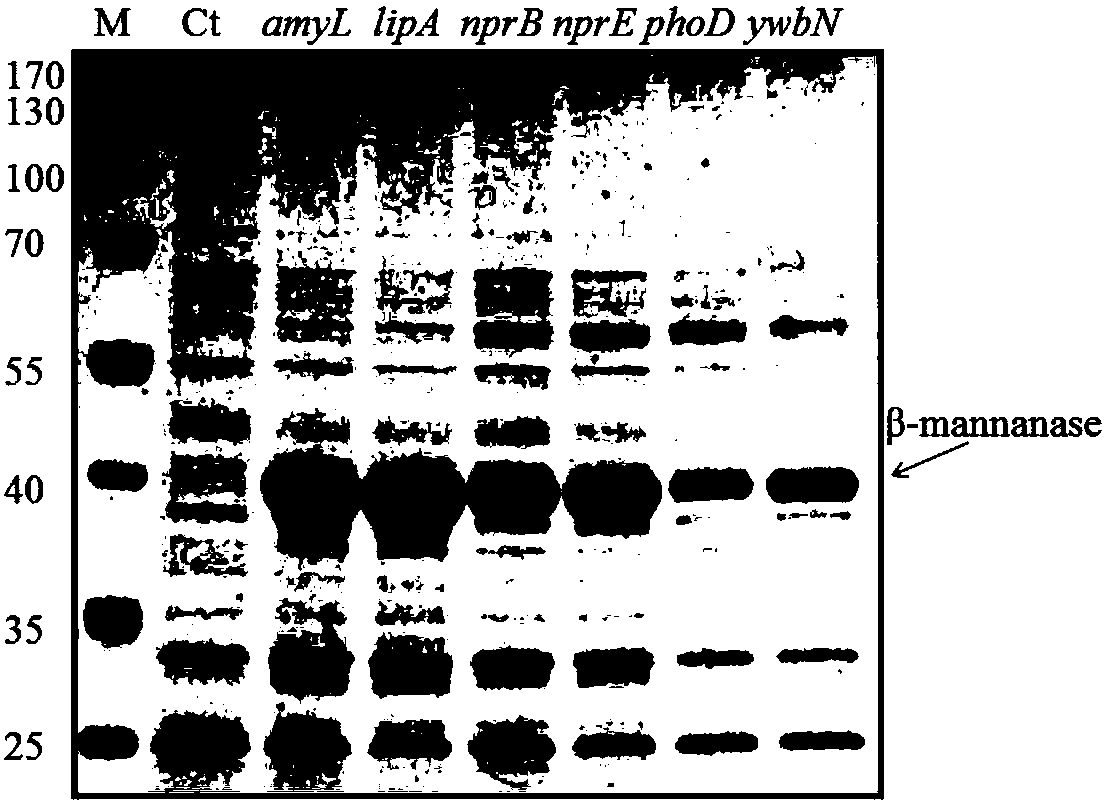
[0041] The β-mannanase gene in the expression vector pMA5 used in this example is derived from Bacillus licheniformis DSM13 and does not contain the original signal peptide DNA sequence. After codon optimization, its nucleotide sequence is the sequence SEQ ID NO: 1. The signal peptide DNA fragments used were derived from amyL (KEGG BSU03040), lipA (KEGG BSU32330), nprB (KEGGBSU11100), nprE (KEGG BSU14700) and phoD (KEGG BSU02620) of the Sec secretion pathway of Bacillus subtilis (Bacillus subtilis 168) and Tat secretion pathway. ), ywbN(KEGGBSU38260).
[0042] 1. Construction of expression vectors containing different signal peptide sequences.
[0043] First, the codon-optimized β-mannanase gene sequence (SEQ ID NO: 1) was cloned into the pMA5 vector (derived from BGSC) by overlapping extension PCR (POE-PCR). On this basis, the Overlap extension PCR (POE-PCR) method, the carrier pMA5 containing the codon-optimized β-mannanase gene sequence and different signal peptide DNA fr...
Embodiment 2
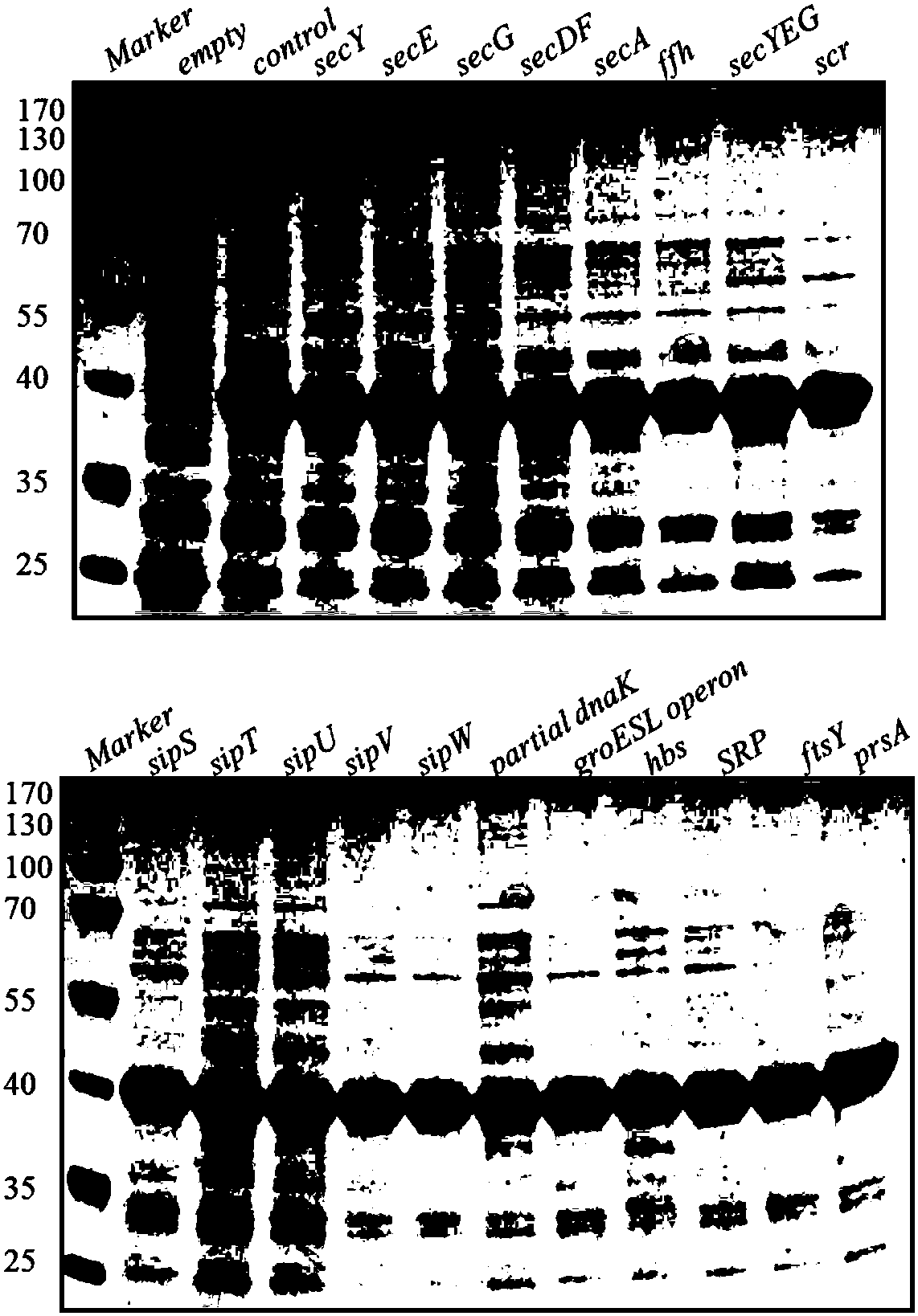
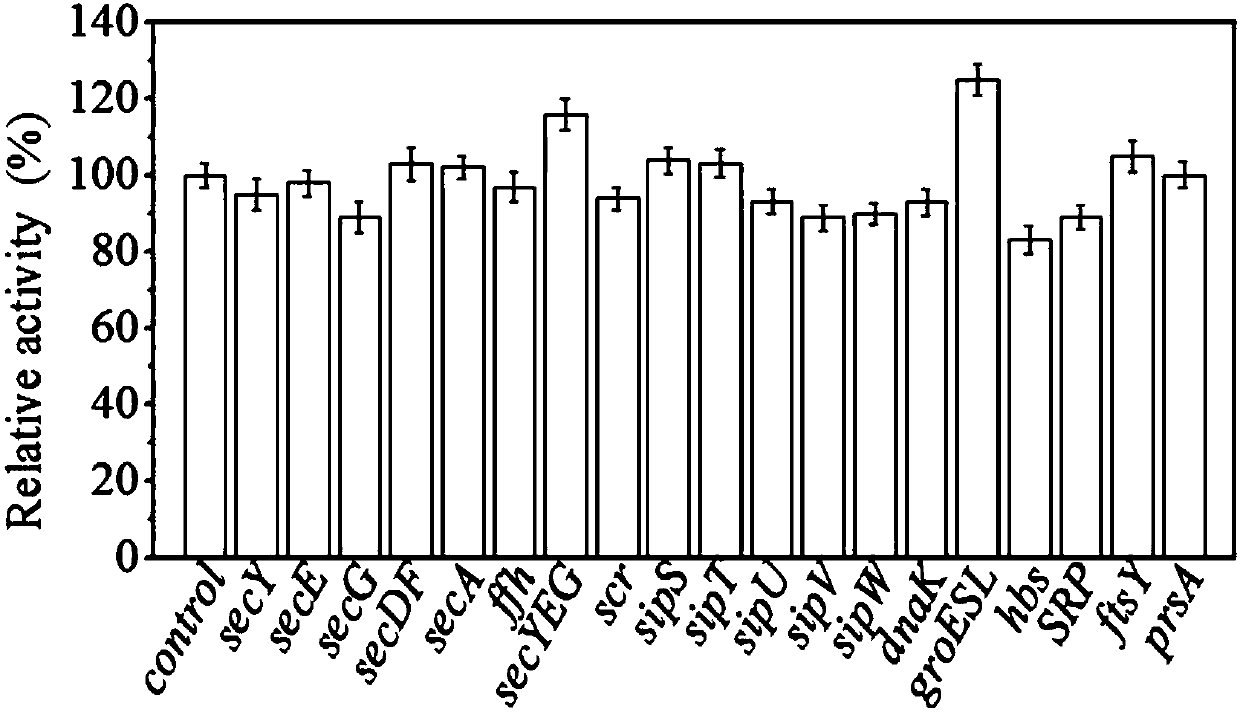
[0069] The expression vector and signal peptide sequence of the β-mannanase in this example are pMA5-2Man and lipA, respectively. The different Bacillus subtilis host bacteria used in this example overexpressed the relevant elements in the Sec secretion pathway in a single copy form on the genome, as well as genes such as signal peptidase and molecular chaperones that may affect the secretion efficiency of the pathway. The Bacillus subtilis host bacteria used to express the β-mannanase gene and the corresponding overexpressed genes are: B. subtilis 1A751S1 (secY), 1A751S2 (secE), 1A751S3 (secG), 1A751S4 ( secYEG), 1A751S5(secDF), 1A751S6(secA), 1A751S7(ffh), 1A751S8(scr), 1A751S9(hbs), 1A751S10(SRP), 1A751S11(ftsY), 1A751P1(sipS), 1A751P 2(sipT), 1A (sipU), 1A751P4(sipV), 1A751P5(sipW), 1A751P6(dnaK / J), 1A751P7(groESL) and 1A751P8(prsA). The overexpression of the above-mentioned genes was integrated into the amyE site of the Bacillus subtilis genome in a single copy form thro...
Embodiment 3
[0077] The expression vector of the β-mannanase used in this example is pMA5-2Man, the promoter it contains is the constitutively expressed hpaII, and the signal peptide is lipA. Using the method described in Example 1, the promoter hpaII Replaced with constitutive promoter fragments aprE and P43 derived from Bacillus subtilis strain 168. The other three inducible promoters used were xylose-inducible promoter xyl (derived from gene BSU17600), IPTG-inducible promoter grac (derived from gene BSU06020) and maltose promoter mglv (derived from gene BSU08180).
[0078] The primer sequences used in this example are listed in Table 4.
[0079] Primer sequences used in table 4 embodiment 3
[0080]
[0081]
[0082] The plasmids and bacterial strains constructed in this example and their characteristics are shown in Table 5.
[0083] Plasmids and bacterial strains constructed in table 5 embodiment 3 and their characteristics
[0084]
[0085] 1. Determination of the concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com