One-step method used for synthesis of iron-based molecular sieve catalyst, and applications thereof
A molecular sieve and catalyst technology, applied in the field of chemistry, can solve problems such as disadvantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
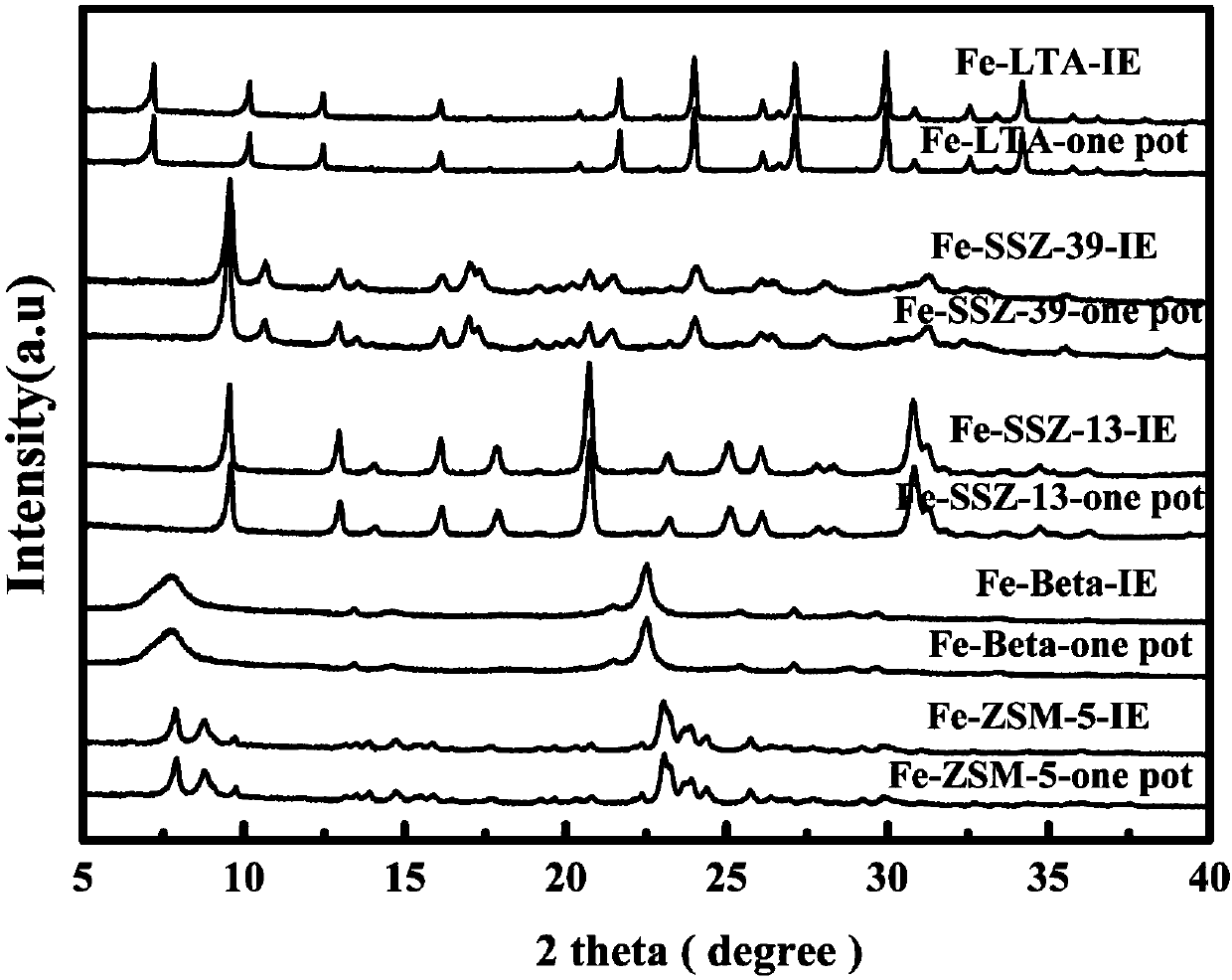
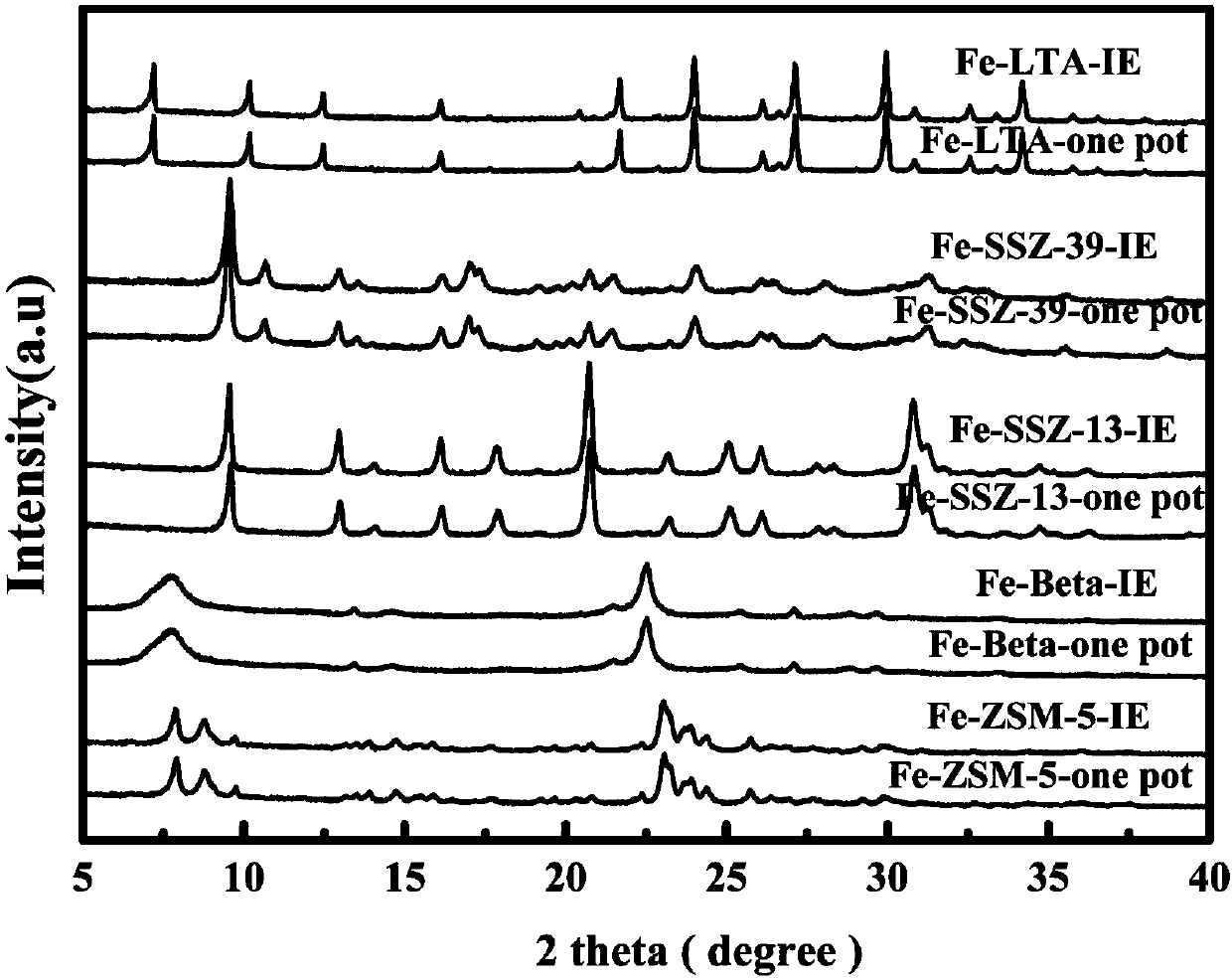
Image
Examples
Embodiment 1
[0061] Embodiment 1: Synthesis of Fe-ZSM-5 molecular sieve catalyst
[0062] Dissolve 0.9g of sodium hydroxide in 22g of deionized water, add 0.51g of sodium metaaluminate after it is fully dissolved, and stir to fully dissolve to obtain solution A. Dissolve 15g of silica sol in 10g of deionized water, then add 3g of tetrapropylammonium bromide, stir thoroughly to obtain solution B, and slowly add solution B to solution A under stirring. Dissolve 0.33g disodium ethylenediaminetetraacetic acid in 10g deionized water, add 0.27g ferrous sulfate after it is completely dissolved, obtain Fe-complex solution after fully complexing, and prepare Fe-complex The solution was slowly added in batches to the mixed solution of A and B, stirred for 4h and then left to stand for 24h. Then it was loaded into a kettle and crystallized at 170°C for 48h. The obtained product was washed with deionized water, suction filtered, dried at 110°C for 12 hours, and then calcined at 600°C for 6 hours to ...
Embodiment 2
[0063] Embodiment 2: Synthesis of Fe-Beta molecular sieve catalyst
[0064] Dissolve 1.68g of sodium hydroxide in 24g of deionized water, add 0.35g of sodium metaaluminate after it is fully dissolved, and stir to fully dissolve to obtain solution A. Dissolve 3.6g of fumed silica in 10g of deionized water, then add 2.56g of tetrapropylammonium bromide, stir well to obtain solution B, and slowly add solution B into solution A while stirring. Dissolve 0.33g disodium ethylenediaminetetraacetic acid in 10g deionized water, add 0.27g ferrous sulfate after it is completely dissolved, obtain Fe-complex solution after fully complexing, and prepare Fe-complex The solution was slowly added in batches to the mixed solution of A and B, and stirred for 4h. Then it was loaded into a kettle and crystallized at 120°C for 144h. The obtained product was washed with deionized water, filtered with suction, dried at 110°C for 12 hours, and then calcined at 600°C for 6 hours to remove the organic te...
Embodiment 3
[0065] Embodiment 3: Synthesis of Fe-SSZ-13 molecular sieve catalyst
[0066] Dissolve 0.81 g of sodium hydroxide in 25.3 g of deionized water, add 2 g of sodium metaaluminate after it is fully dissolved, and stir to fully dissolve to obtain solution A. Dissolve 20g of silica sol in 10g of deionized water, then add 13.55g of N,N,N-trimethyl-1-adamantyl ammonium hydroxide, stir thoroughly to obtain solution B, and slowly add solution B to A solution. Dissolve 0.66g disodium ethylenediaminetetraacetic acid in 10g deionized water, add 0.56g ferrous sulfate after it is completely dissolved, obtain Fe-complex solution after fully complexing, and prepare Fe-complex The solution was slowly added in batches to the mixed solution of A and B, and stirred for 4h. Then put it in a kettle and crystallize at 150°C for 96h. The obtained product was washed with deionized water, filtered with suction, dried at 110°C for 12 hours, and then calcined at 600°C for 6 hours to remove the organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com