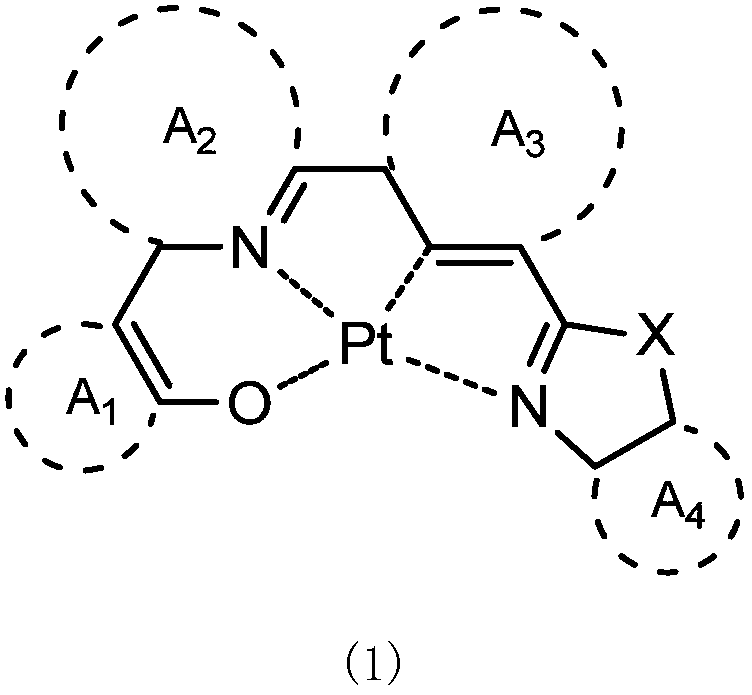
Tetradentate platinum (II) complex material based on oxazole, thiazole or imidazole and its application
A complex, thiazole technology, applied in the field of phosphorescent doping materials, can solve the problems of poor stability and performance to be improved, and achieve the effects of low efficiency roll-off, low quenching constant, and high fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
[0066] Synthesis of Compound 1: Dissolve 5.45g (50mmol) of o-aminophenol in 125mL of toluene, add 9.25g (1.0eq., 50mmol) of m-bromobenzaldehyde, stir at room temperature for 1hr, then heat to 110°C for 12hr under reflux. After the reaction stopped, cool to room temperature, remove the solvent by rotary evaporation, then add an appropriate amount of water and ethyl acetate for extraction, collect the organic phase, dry it with anhydrous magnesium sulfate, add an appropriate amount of silica gel, remove the solvent by rotary evaporation, and use n-hexane / ethyl acetate system Through column chromatography, 9.32 g of white solid was obtained, with a yield of 68% and a purity of 99.0%.
[0067] The synthesis of compound 2: get 5.48g (20.0mmol) compound 1, biboronic acid pinacol ester 6.35g (1.25eq., 25.0mmol), potassium carbonate 2.59g (1.25eq., 25.0mmol) and Pd (dppf) Cl 2 292mg (0.02eq., 0.4 mmol), was added to a three-necked flask, vacuumed and replaced with nitroge...
Embodiment 2
[0073]
[0074] Synthesis of Compound 5: Dissolve 5.45g (50mmol) of o-aminothiophene in 125mL of toluene, add 9.25g (1.0eq., 50mmol) of m-bromobenzaldehyde, stir at room temperature for 1hr, then heat to 110°C for 12hr under reflux. After the reaction stopped, cool to room temperature, remove the solvent by rotary evaporation, then add an appropriate amount of water and ethyl acetate for extraction, collect the organic phase, dry it with anhydrous magnesium sulfate, add an appropriate amount of silica gel, remove the solvent by rotary evaporation, and use n-hexane / ethyl acetate system Column chromatography yielded 13.05 g of white solid with a yield of 90% and a purity of 99.0%.
[0075] The synthesis of compound 6: get 5.80g (20.0mmol) compound 5, biboronic acid pinacol ester 6.35g (1.25eq., 25.0mmol), potassium carbonate 2.59g (1.25eq., 25.0mmol) and Pd (dppf) Cl 2 292mg (0.02eq., 0.4 mmol), was added to a three-necked flask, evacuated and replaced with nitrogen several ...
Embodiment 3
[0080]
[0081] Synthesis of compound 9: get 5.41g (50mmol) o-aminophenol and dissolve in 150mL acetonitrile, add 9.25g (1.0eq., 50mmol) m-bromobenzaldehyde, stir at room temperature for 1hr, then add 36% hydrochloric acid 16.5mL and 30 % hydrogen peroxide 41.1mL, stirred at room temperature for 12hr. After the reaction stopped, add saturated sodium carbonate solution to adjust the pH to neutral, then add an appropriate amount of water and ethyl acetate to extract, collect the organic phase, dry it over anhydrous magnesium sulfate, add an appropriate amount of silica gel, and remove the solvent by rotary evaporation. The ester system was subjected to column chromatography to obtain 11.74 g of a white solid with a yield of 86% and a purity of 99.0%.
[0082] Synthesis of Compound 10: Take 5.46g (20.0mmol) of Compound 9, dissolve it in 60mL N,N-dimethylformamide, then add 4.80g (6.0eq., 120.0 mmol), after stirring the reaction for 2hr, 3.29 g (24.0 mmol) of n-bromobutane was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com