Synthesis method of radioisotope carbon-14 labeled imidacloprid
A technology of radioisotope and synthesis method, applied in the field of radiochemical synthesis, can solve the problem that the labeling synthesis method has not been publicly reported, and achieve the effects of simple and easy synthesis technical route, high radiochemical purity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
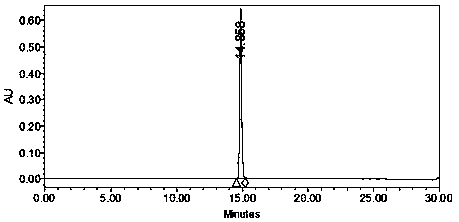
Image
Examples
Embodiment Construction
[0021] The following examples are given to illustrate the present invention. The implementation examples are only used to further illustrate the present invention, and do not represent the protection scope of the present invention. Non-essential modifications and adjustments made by others according to the present invention still belong to the protection scope of the present invention.
[0022] At room temperature and under the protection of argon, add anhydrous lithium chloride (420 mg) into the reaction flask, heat with a heat gun, and repeat the vacuum-filling operation 5 times to thoroughly dry the lithium chloride. Add magnesium chips (480 mg), replace with argon three times, add anhydrous THF (10 mL), and stir until lithium chloride is completely dissolved. Add diisobutylaluminum hydride (1 M, 160 μL) and stir at room temperature for 5 min. Cool down to 0°C, slowly add 2-chloro-5-bromopyridine (1.540 g) anhydrous THF solution (10 mL) dropwise, and continue stirring unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com