Antibody prodrug conjugate and preparation and use thereof
A technology of antibody prodrugs and conjugates, which is applied in the field of preparation of antibody prodrug conjugates, can solve the problems of bystander toxicity, poor metabolic stability, etc., achieve good tumor treatment effect, low toxicity and side effects, and enhance tumor tissue The effect of penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Synthesis of doxorubicin prodrug (TCO-Dox-Mal) protected by trans-cyclooctene in response to tumor microenvironment pH The synthetic route of TCO-Dox-Mal is as follows:
[0044]
[0045]
[0046] (1) Synthesis of compound 1
[0047]Add cyclooctene-2-alcohol ((Z)-cyclooct-2-en-1-ol) (8.5g, 67mmol), methyl benzoate (9.2g, 67mmol), 500mL to 1L quartz bottle Anhydrous ether and 1000mL n-hexane. The reaction solution was subjected to light reaction for 24 hours, the wavelength of the light source was 254nm, and the intensity was 300W. During the photoreaction, the reaction system was circulated through a peristaltic pump, and the product was enriched through a silver nitrate silica gel column (the mass fraction ratio of silver nitrate and silica gel was 25:200), and the flow rate of the peristaltic pump was 18 mL / min. After the reaction, the silver nitrate silica gel was taken out, 500 mL of ammonia water was added and stirred for 5 min, and then extracted...
Embodiment 2
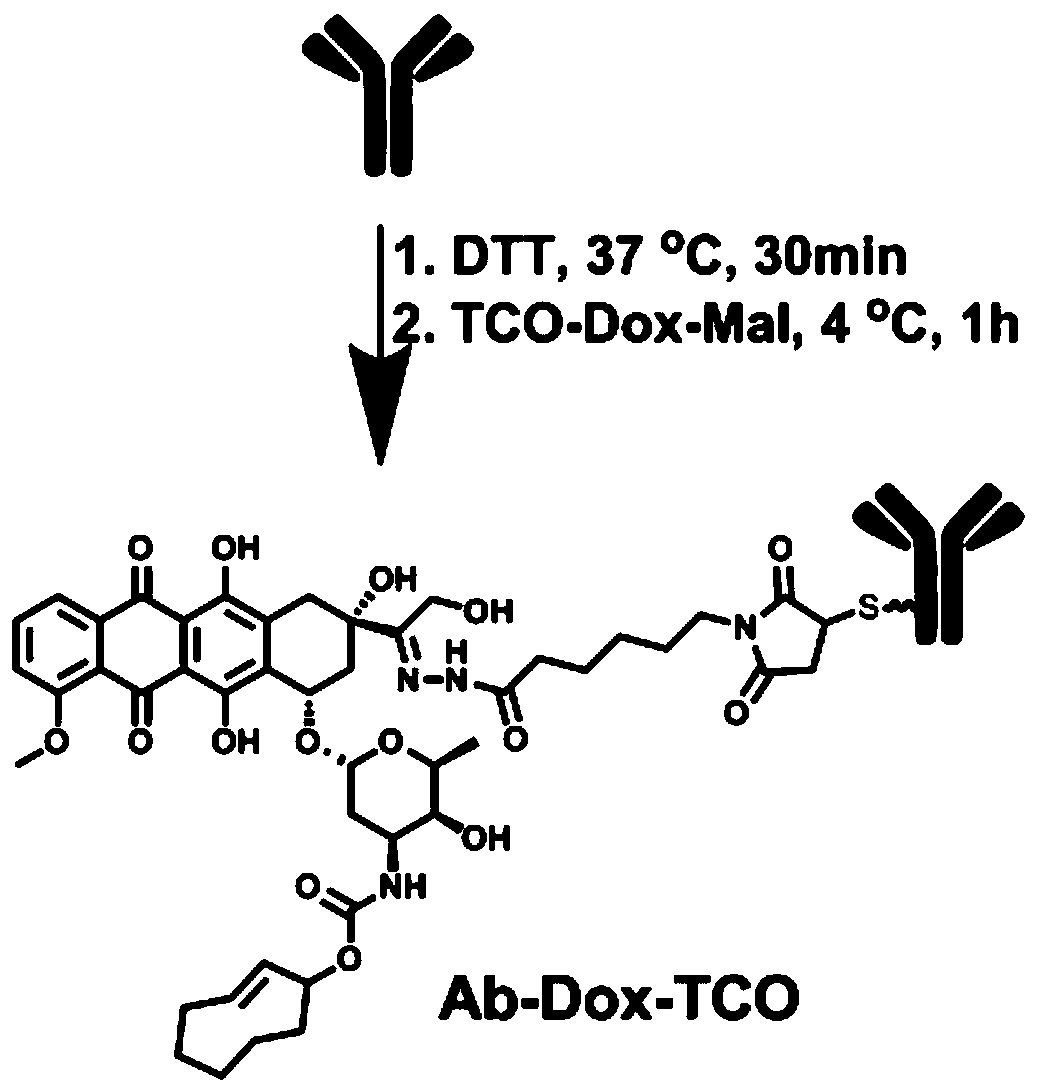
[0054] Example 2. Preparation of Antibody Prodrug Conjugate Ab-Dox-TCO and Determination of DAR
[0055] For the preparation process of antibody prodrug conjugates, see figure 1 . Dissolve 10 mg of trastuzumab monoclonal antibody in 10 mL of PBS solution, and then add DTT (33.3 μL, 3.33 μmol) for reduction at 37° C. for 30 min. After the reaction, the reduced trastuzumab monoclonal antibody was desalted with a PD 10 desalting column, and then centrifugally concentrated to 10 mg / mL for use. Prepare 100 mM TCO-Dox-Mal DMSO solution, take 33.3 μL and add it to the above 10 mg / mL reduced monoclonal antibody solution. Then the reaction system was carried out at 4° C. for 1 h. After the end, repeated desalting and concentration were carried out to finally obtain the antibody prodrug conjugate. Wherein, the concentration of the antibody prodrug conjugate was measured by A280 on the Nanodrop (1 mg / mL=1.4AU). The concentration of Dox is obtained by absorbing light at 495nm on the ...
Embodiment 3
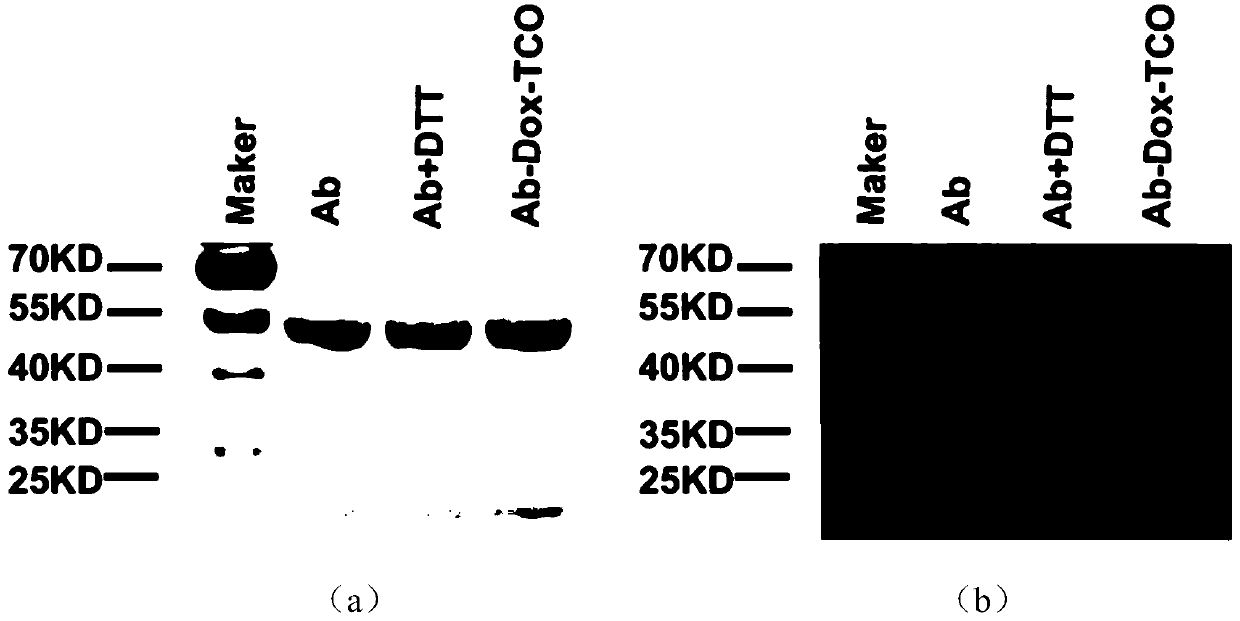
[0056] Example 3. SDS-PAGE Characterization of Antibody Prodrug Conjugates
[0057] Take 16 μL of 10 mg / mL antibody prodrug conjugate Ab-Dox-TCO and antibodies of the same volume and concentration, add 4 μL of SDS-PAGE loading buffer respectively, and then boil the sample at 95°C for 30 minutes to fully denature the protein. Then take 10 μL of the boiled sample for SDS-PAGE electrophoresis. Electrophoresis conditions are 12% polyacrylamide gel, 100V voltage for 1h. After the end, the gel was stained with Coomassie Brilliant Blue, and after decolorization, it was developed with ChemiDoc XRS+molecular imager (molecular imager), as shown in figure 2 shown. The channels used are Comassie channel and fluorescence channel(exct:488nm).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com