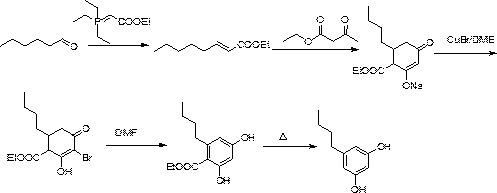
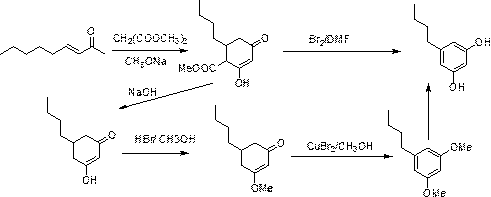
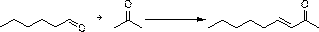3,5-dihydroxyamylbenzene synthesis method
A technology of hydroxypentylbenzene and synthetic methods, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low raw material cost, cumbersome synthesis, difficult synthesis, etc., and achieve the effect of low price and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] The present invention discloses a synthetic method of 3,5-dihydroxypentylbenzene, comprising the following steps,
[0076] S1, using benzene and n-valeryl chloride as raw materials to perform Friedel-crafts acylation reaction under Lewis acid catalysis to obtain valerophenone;
[0077] S2. The valerophenone obtained in S1 is subjected to a nitration reaction with a nitrating agent to obtain 3,5-dinitrovalerophenone;
[0078] S3. Under the action of sodium borohydride and strong carboxylic acid, the 3,5-dinitrovalerophenone obtained in S2 is reduced to methylene from the carbonyl group to form 3,5-dinitropentylbenzene; ,5-Dinitropentylbenzene is reduced to 3,5-diaminopentylbenzene under the action of iron powder and ammonium chloride;
[0079] S4, diazotizing the 3,5-diaminopentylbenzene obtained in S31 under the action of sulfuric acid and sodium nitrite to form 3,5-dihydroxypentylbenzene.
[0080] The above reaction formula is as follows:
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com