Porphyrin-chrysin compounds and anti-tumor activity thereof
A technology of porphyrin and compound, which is applied in the field of porphyrin-chrysin complex, can solve the problems of poor water solubility, easy metabolism and inactivation, and achieve the effect of broadening the scope and reducing the side effects of killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Synthesis of porphyrin-chrysin complex
[0038] .
[0039] Add 2 (110.1 mg) / 3 (118.1 mg) / 4 (101.8 mg), (0.15 mmol) to a 100 mL three-necked flask, add an appropriate amount of baked potassium carbonate and potassium iodide as a catalyst, and then add 40 mL of DMF As a solvent, the reaction was stirred and refluxed at 80°C (65°C, 4). After 30 min, chrysin derivatives 1a (1b, 1c, 1d, 1e) (0.18 mmol) were added, and the reflux reaction was continued for about 8 h. The reaction was monitored by TLC until the raw material point remained unchanged. Pour the cooled reaction solution into a separatory funnel, extract the reaction product with dichloromethane solvent, and wash with water several times to remove impurities such as DMF solvent, potassium carbonate, and potassium iodide. After removing water from the organic solvent, the solvent was spin-dried by a rotary evaporator, and the remaining solid was separated and purified by silica gel column chromatograph...
Embodiment 2
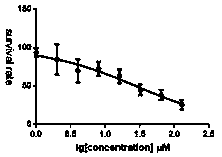
[0054] Example 2 MTT colorimetric assay for in vitro anti-tumor cell activity
[0055] Cell inoculation: Digest gastric cancer cell MGC-803 and cervical cancer cell Hela cultured in monolayer with 0.25% trypsin, respectively, and use 10% concentration of calf serum (containing 1×10 5 U·L -1 Penicillin and 1×10 5 U·L -1 Streptomycin) RPMI-1640 culture medium to culture cells in 96-well culture plate at 5×10 per well 3 A cell suspension was inoculated with a volume of 150 µL per well. Only the same amount of culture medium was added to the blank group. And add a blank PBS solution to the outermost circle of the inoculation plate. Culture cells: transfer the seeded 96-well plate to 37 ℃, 5% CO 2 The incubator continued to cultivate for about 48 h until the cell monolayer covered the bottom of the well.
[0056] Carefully suck off the supernatant in the wells of the 96-well plate with a pipette gun, and add 150 μL of the compound solution to be tested prepared in advance ...
Embodiment 3
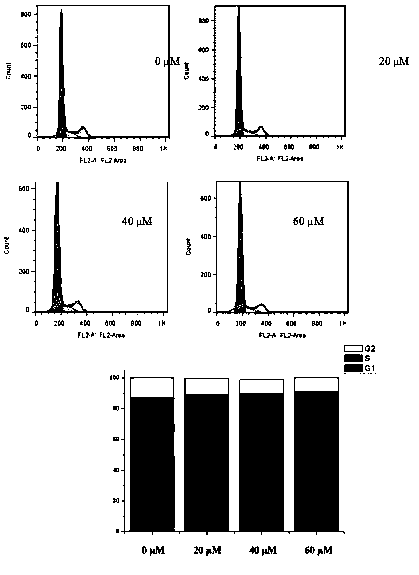
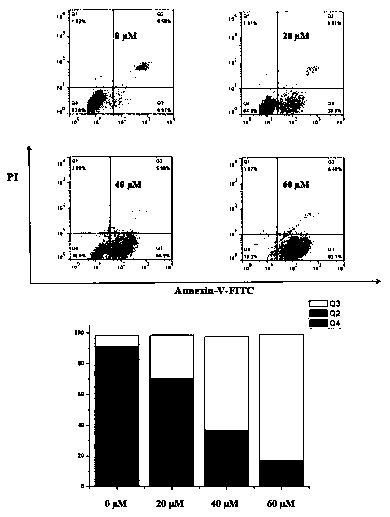
[0063] Example 3 Cell cycle distribution experiment
[0064] Dosing: Hela cells were seeded on a 6-well plate at about 100,000 cells per well, and placed in an incubator for 24 hours. With the drug (4a) concentration gradient of 0 (Control), 20, 40, and 60 μM, set up 2 auxiliary wells for each concentration, add the drug, and continue to cultivate for 48 h. After the first 4 hours, the orifice plates with 4 concentrations were taken out, and the 12 W LED purple light was used to continuously irradiate for 10 min at a distance of 20 cm from the 6-well plate, and then put into the incubator to continue culturing.
[0065] Fixation: Aspirate the drug-containing medium and wash with PBS, digest the cells with trypsin, centrifuge the cell suspension, and wash three times with PBS. Add 1 mL of 70% ethanol at 4 °C to each sample, resuspend the cells, cover the lid, and place in a 4 °C environment for 12 h.
[0066] Staining: After 12 h, centrifuge and wash twice in the same way. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com