Preparation method and product of tin dioxide doped hydrosol and application thereof in self-cleaning of cotton fabrics
A tin dioxide, hydrosol technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, water pollutants, etc., can solve the problems of fabrics that are not easy to bond, easy to aggregate, and small particles, etc. To achieve the effect of improving the binding force of nanoparticles and fabrics, increasing electrostatic attraction, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
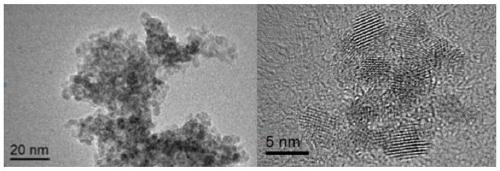
[0035] Preparation of doped tin dioxide crystals: Weigh an appropriate amount of tin powder 0.36g (0.003mol) and crystalline tin tetrachloride 0.7g (0.002mol), add 60mL of distilled water, stir and add to the stainless steel with Teflon lining In the reaction kettle, bake in an oven at 180°C for 24 hours, after cooling for 5 hours, remove the supernatant, put the lower layer in a centrifuge tube, centrifuge at 10,000 rpm for 5 minutes, take the precipitate, add 8 mL of distilled water, wash once, add 8 mL of ethanol, After washing the precipitate for 3 times, centrifuge at 10000rpm for 5 minutes, dry the precipitate in an oven at 60°C for 30 minutes, place it in a grinding container and grind it to obtain the doped tin dioxide crystal;
[0036] Preparation of doped tin dioxide hydrosol: Weigh 8 mg of 3,4-dihydroxyphenylacetic acid, dissolve it in 50 mL of distilled water, ultrasonicate at 50 KHz, 30 °C for 5 min, stir with a magnetic stirrer at 700 rpm for 10 min, add 0.5 g dop...
Embodiment 2
[0042] Preparation of doped tin dioxide crystals: Weigh an appropriate amount of tin powder 0.36g (0.003mol) and crystalline tin tetrachloride 0.7g (0.002mol), add 60mL of distilled water, stir and add to the stainless steel with Teflon lining In the reaction kettle, bake in an oven at 180°C for 24 hours, after cooling for 5 hours, remove the supernatant, put the lower layer in a centrifuge tube, centrifuge at 10,000 rpm for 5 minutes, take the precipitate, add 8 mL of distilled water, wash once, add 8 mL of ethanol, After the precipitate was washed 3 times, it was centrifuged at 10,000 rpm for 5 minutes, dried in an oven at 60° C. for 30 minutes, and ground in a grinding container to obtain the doped tin dioxide crystal.
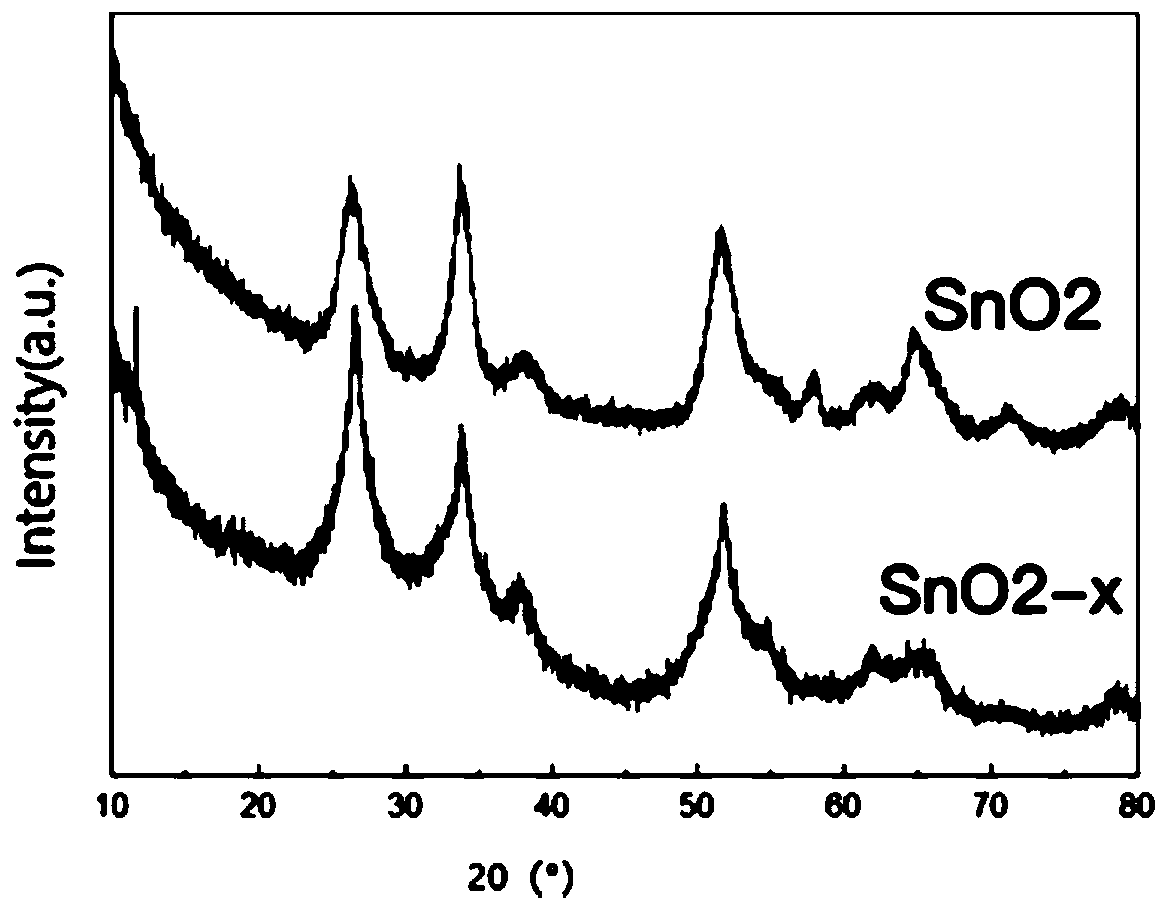
[0043] In order to find a better catalyst formula, tin powder and tin tetrachloride in different molar ratios were studied, and their photocatalytic activity was measured, see Figure 5 . It can be seen from the figure that the best degradation rate is the...
Embodiment 3
[0046] Cationic modification of cotton fabric: Weigh 2 grams of cotton fabric, bath ratio 1:30, weigh 3 grams of 3-chloro-2-hydroxypropyltrimethylammonium chloride according to the concentration of 50g / L, and weigh 30g / L Put 0.9 g of L sodium hydroxide into a conical flask. Vibrate for about one hour at 80°C in an oscillating dyeing machine, wash with distilled water, and dry in an oven at 60°C.
[0047] Cotton fabric finishing: add the cationic modified cotton cloth to the modified self-doped tin dioxide hydrosol solution, dipping and rolling twice, the excess rate is 100%, after slight water washing, dry in an oven at 60°C.
[0048] Figure 6 It is the SEM picture of cotton fabric, wherein, (1) original cloth; (2) original cloth; (3) cotton cloth doped with tin dioxide; (4) cotton cloth doped with tin dioxide. It can be seen that the shape of the fiber is a flat ribbon structure with twists and turns, which conforms to the morphology characteristics of cotton fiber. After...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com