Method for measuring Farnesal contents in medicine loading micelle by high performance liquid chromatography
A technology of high performance liquid chromatography and drug-loaded micelles, which is applied in the field of drug analysis to achieve the effects of strong specificity, easy operation, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
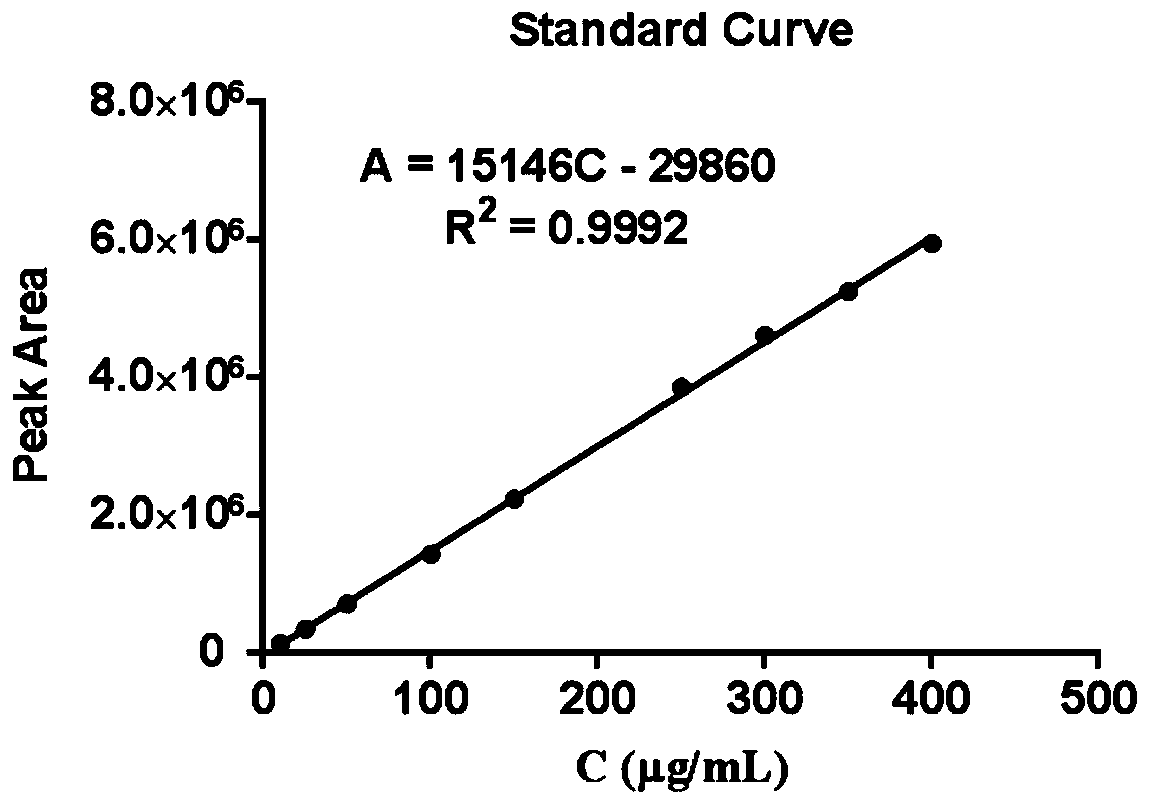
[0046] The method for determining the content of farnesaldehyde in drug-loaded micelles by high performance liquid chromatography of the present embodiment adopts Agilent-1260 high performance liquid chromatography, comprising the following steps:
[0047] (1) Selection of detection wavelength
[0048] Accurately weigh 5 mg of farnesal (Farnesal, Far), place it in a 25 mL volumetric flask, add acetonitrile to dissolve it completely, and constant volume to obtain farnesal stock solution (100 μg / mL). Measure a small amount of farnesaldehyde stock solution, dilute it with acetonitrile to an appropriate concentration, and use acetonitrile as a blank control, and use a UV spectrophotometer to scan in the wavelength range of 200-600nm to determine the maximum absorption wavelength.
[0049] Through the full-wavelength scanning of the ultraviolet spectrophotometer, 216nm was selected as the UV detection wavelength.
[0050] (2) Chromatographic conditions
[0051] In this experiment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com