Improved fluorescent protein transgenic mouse tissue fluorescence imaging method
A transgenic mouse, fluorescent protein technology, applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of difficult localization of GFP fluorescent protein, loss of tissue, hindering popularization and application, etc., to make up for the loss of signal in tissue slices, to avoid deterioration and fragmentation , the effect of improving the efficiency of nuclear staining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The features and advantages of the present invention can be further understood through the following detailed description in conjunction with the accompanying drawings. The examples provided are only illustrative of the method of the present invention and do not limit the rest of the present disclosure in any way. [Example 1] The process of determining the fixed temperature, fixed time, Triton concentration, nuclear staining dye and nuclear staining time in the fluorescence imaging method of GFP transgenic mouse tooth germ tissue is as follows:
[0039] (1) Fixed temperature and fixed time: Fix the incisor terminal tissue pieces of P2GFP transgenic mice in 4% paraformaldehyde at room temperature or 4 degrees for 1 minute, 2 minutes, 5 minutes, 10 minutes, 30 minutes, 60 minutes respectively . In the subsequent counterstaining and imaging process, the tissue integrity and GFP fluorescence signal intensity are the best when the tissue is fixed at 4 degrees for 5 minutes;...
Embodiment 2
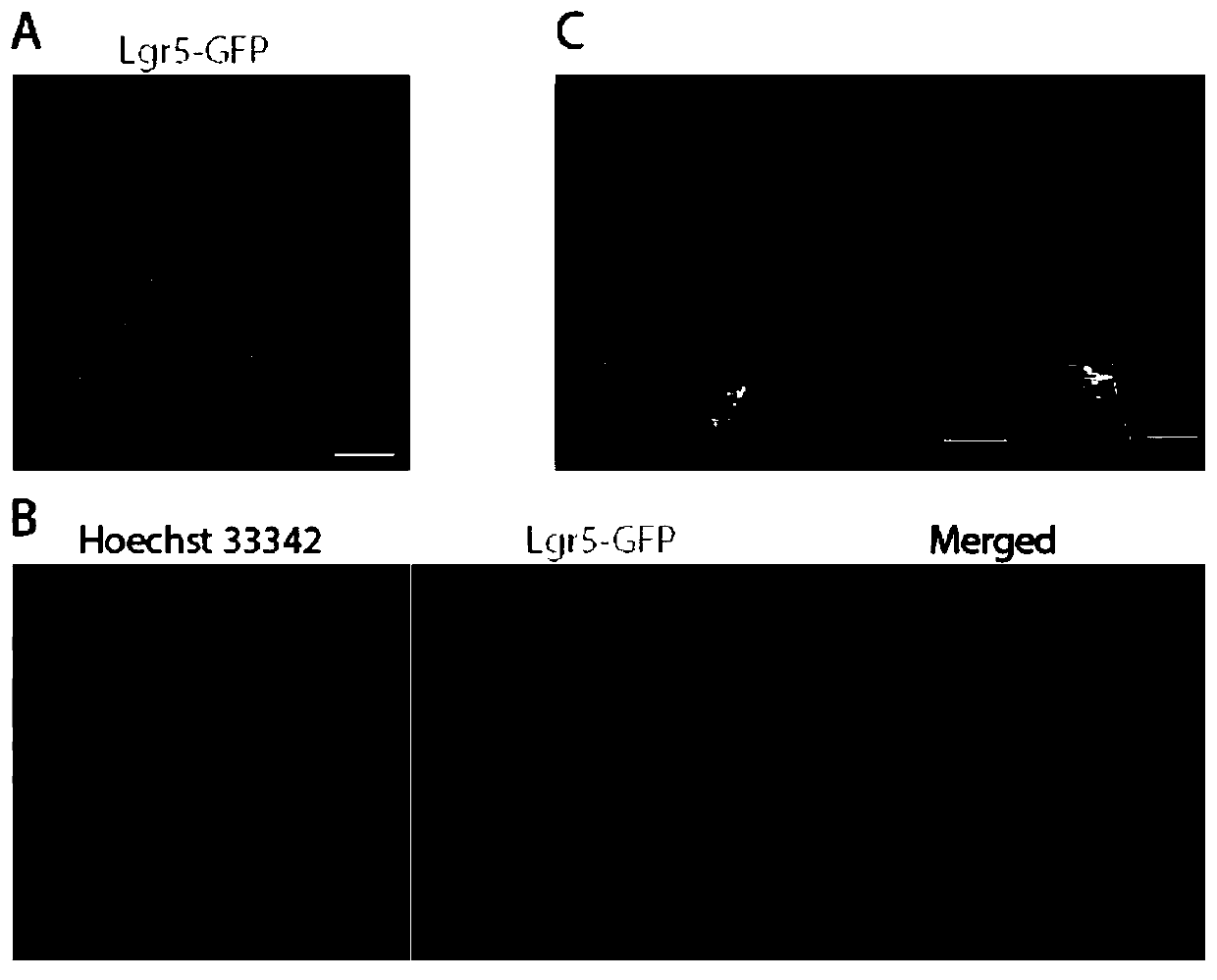
[0042] [Example 2] Confocal fluorescence imaging of incisor tooth germ tissues of Lgr5-GFP transgenic mice
[0043] 1. Materials and methods
[0044] 1.1 Materials
[0045] Animals: one litter (6-8) of Lgr5-GFP transgenic mice 2 days after birth (P2);
[0046] Reagents: 1×PBS, 1×PBS-0.5% Triton, 10mg / mL Hoechst 33342, Vestsheild;
[0047] Consumables and instruments: glass slides, coverslips, confocal microscope.
[0048] 1.2 Method
[0049] (1) Dissect and obtain the incisor terminal tissue pieces of Lgr5-GFP transgenic P2 mice, rinse slowly with shaking in 1×PBS at room temperature for 5 minutes×2 times, soak in 4% paraformaldehyde, and place at 4°C for slow shaking , fix for 5 minutes, after fixation, slowly shake and rinse in 1×PBS at room temperature for 5 minutes×2 times;
[0050] (2) Soak the fixed tissue block in 1×PBS-0.5% Triton, shake slowly and rinse for 5 minutes×2 times;
[0051] (3) Soak the above-treated tooth germ tissue block in the fluorescent dye Hoes...
Embodiment 3
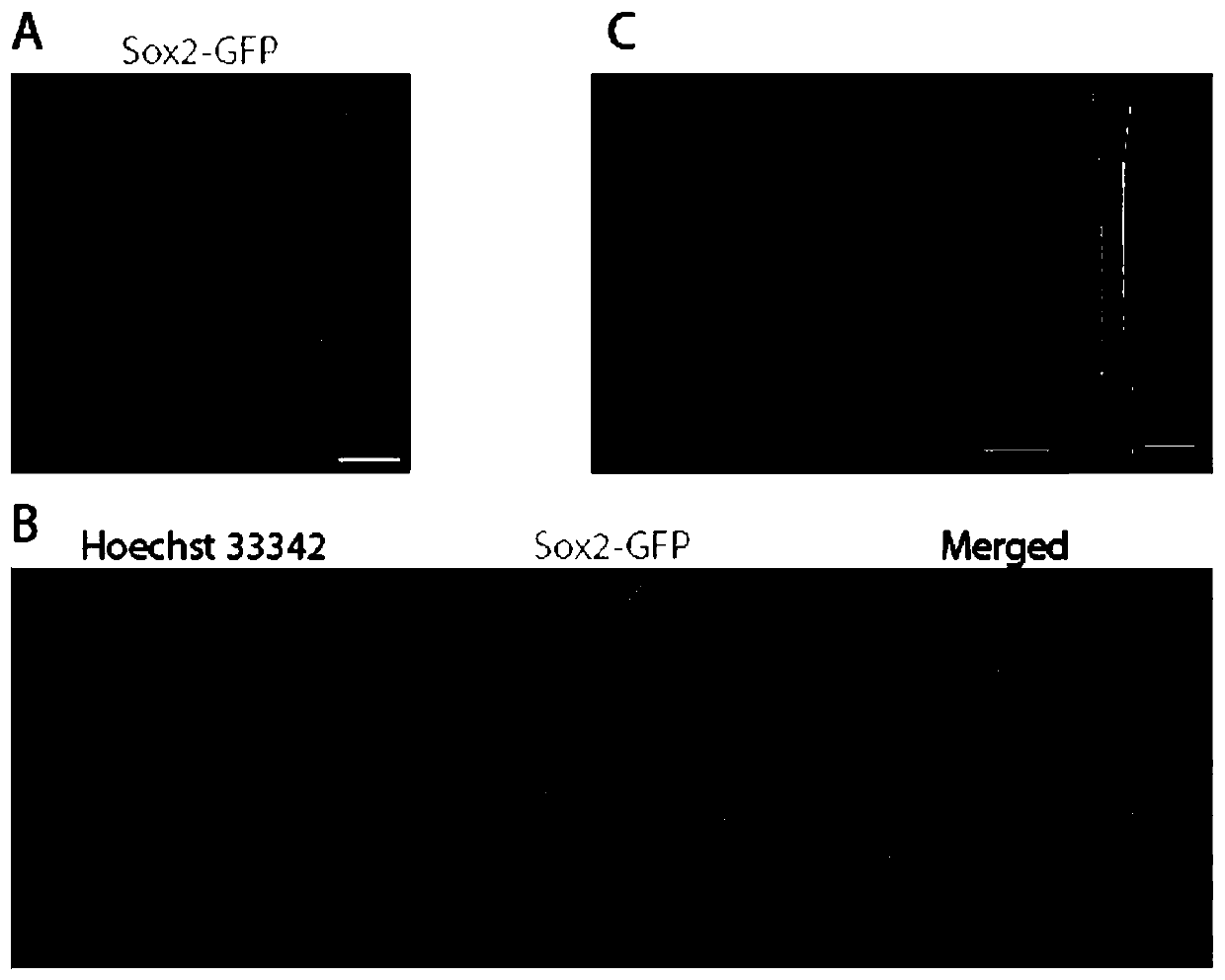
[0057] [Example 3] Confocal fluorescence imaging of incisor tooth germ tissue in Sox2-GFP transgenic mice
[0058] 1. Materials and methods
[0059] 1.1 Materials
[0060] Animals: one litter (6-8) of Sox2-GFP transgenic mice 2 days after birth (P2);
[0061] Others are with embodiment 1.
[0062] 1.2 Method
[0063] (1) Dissect and obtain end tissue pieces of incisors from Sox2-GFP transgenic P2 mice.
[0064] (2) Others are the same as in Embodiment 1.
[0065] (4) Results
[0066] The blue-stained nuclei (Hoechst33342 staining) are clearly visible, making the stem cell nest structure easy to identify; the green Sox2-GFP signal is evenly distributed in most cells of the entire stem cell nest, and it shows obvious nuclear and plasma expression patterns.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com