Preparation method and application of Z-type photocatalyst MgAl LDH/CN-H
A photocatalyst, g-c3n4 technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problem of high photogenerated electron-hole recombination rate, narrow light absorption range, and poor utilization Advanced problems, to achieve the effect of improving the separation rate of photogenerated carriers and increasing the comparison area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034](1) Preparation of CN-H materials
[0035] Melamine was placed in a crucible with a lid, heated to 500°C in a muffle furnace at a rate of 2°C / min, kept for 2 hours, and then heated to 520°C for 2 hours. After cooling to room temperature, the yellow product was collected and ground into powder to obtain g-C 3 N 4 (marked as CN). 1g g-C 3 N 4 Disperse in 200ml of HNO 3 (8mol / L) solution, and refluxed at 90°C for 3h, cooled to room temperature, centrifuged and washed several times, and dried at 80°C overnight, and the sample powder was obtained after grinding, and the acidified g-C 3 N 4 Samples are labeled CN-H.
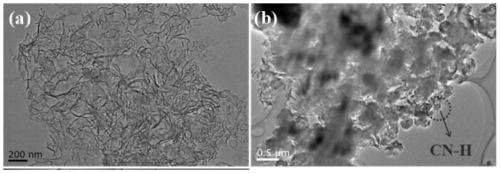
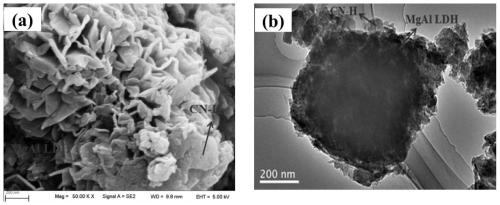
[0036] g-C 3 N 4 and TEM images of CN-H see figure 1 . From figure 1 It can be seen that the original g-C 3 N 4 It presents a thin layer with wrinkles (a layer with a large two-dimensional size), while CN-H presents a small flake shape, and there are relatively many pores between the small flakes. show that the acidification process makes g-C 3 N ...
Embodiment 2
[0044] (1) Preparation of CN-H materials
[0045] Melamine was placed in a crucible with a lid, heated to 500°C in a muffle furnace at a rate of 2°C / min, kept for 2 hours, and then heated to 520°C for 2 hours. After cooling to room temperature, the yellow product was collected and ground into powder to obtain g-C 3 N 4 (marked as CN). 1g g-C 3 N 4 Disperse in 200ml of HNO 3 8mol / L) solution, and refluxed at 95°C for 2h, cooled to room temperature, centrifuged and washed several times, and dried at 80°C overnight, and the sample powder was obtained after grinding, and the acidified g-C 3 N 4 Samples are labeled CN-H.
[0046] (2) Preparation of MgAl LDH material
[0047] 0.252g citric acid and 1.21g urea were added to the mixed solution of 60mL ethanol and water (V 乙醇 :V 水 =1:1), stirred for 30 minutes. Then add 1.026g of Mg(NO 3 ) 2 ·6H 2 O and 0.75g Al(NO 3 ) 3 9H 2 O, after being completely dispersed in the above solution, hydrothermally reacted at 160°C for...
Embodiment 3
[0061] (1) Preparation of CN-H materials
[0062]Melamine was placed in a crucible with a lid, heated to 500°C in a muffle furnace at a rate of 2°C / min, kept for 2 hours, and then heated to 520°C for 2 hours. After cooling to room temperature, the yellow product was collected and ground into powder to obtain g-C 3 N 4 (marked as CN). 1g g-C 3 N 4 Disperse in 200ml of HNO 3 (4mol / L) solution, and refluxed at 100°C for 1h, cooled to room temperature, centrifuged and washed several times, and dried at 80°C overnight, and the sample powder was obtained after grinding, and the acidified g-C 3 N 4 Samples are labeled CN-H.
[0063] (2) Preparation of MgAl LDH material
[0064] 0.126g citric acid and 0.605g urea were added to the mixed solution of 60mL ethanol and water (V 乙醇 :V 水 =1:1), stirred for 30 minutes. Then add 0.513g of Mg(NO 3 ) 2 ·6H 2 O and 0.375g Al(NO 3 ) 3 9H 2 O, after being completely dispersed in the above solution, hydrothermally reacted at 180°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com