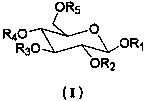
Aromatic-substituted glucose-like compound as well as preparation method of pharmaceutical compositions thereof and analgesia application of compound
A compound and drug technology, applied in the field of natural medicinal chemistry, can solve the problems of no analgesic activity and achieve good analgesic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Extraction, separation and purification of compound 1-19 of the present invention:
[0030] will thick skin fragrant [ Ternstroemia gymnanthera (Wight et Arn.) Bedd.] The aerial part (14kg) of the plant was dried in the shade, crushed to 30 mesh, extracted three times with 95% ethanol at room temperature, 60 L each time, 24 h, combined the extracts, and reduced pressure Concentrate the extract to obtain the extract, suspend it with appropriate amount of water, and distribute it with ethyl acetate for 6 times to obtain ethyl acetate extract (726 g), dissolve the extract with appropriate amount of chloroform / acetone and mix the sample with silica gel 80-100 mesh , and then use 650 g of silica gel 200-300 mesh to carry out column chromatography for rough separation, and carry out gradient elution with chloroform / methanol (1 : 0-0 : 1) to obtain 7 main fractions. Silica gel column chromatography, 8 : 2 chlorine / A fraction and 7 : 3 chlorine / A fraction were carried out ...
Embodiment 2
[0032] Physical and spectral data of compounds 1-19 of the present invention:
[0033] Compound 1: brown solid. UV (MeOH) lambda max (log ε ): 223 (4.12), 291 (3.58) nm; HR-ESI-MS m / z : 315.1405 [M-H] - (calcd for C 14 h 19 o 8 , 315.1402); 1 H-NMR (400 MHz, CD 3 OD) δ : 6.62 (1H, d, J = 2.1 Hz, H-2'), 6.61 (1H, d, J = 8.1 Hz, H-5'), 6.49 (1H, dd, J = 8.0, 2.1 Hz, H-6'), 4.23 (1H, d, J = 7.8 Hz, H-1), 3.96 (1H, m, H-8’a), 3.80 (1H, dd, J = 11.9, 1.9 Hz, H-6a), 3.62 (2H, overlap, H-6b, 8'b), 3.30 (1H, overlap, H-5), 3.25 (1H, overlap, H-4), 3.21 ( 1H, overlap, H-3), 3.13 (1H, dd, J = 9.0, 7.8 Hz, H-2), 2.71 (2H, m, H 2 -7'); 13 C-NMR (100 MHz, CD 3 OD) δ: 104.3 (d, C-1), 75.0 (d, C-2), 77.8 (d, C-3), 71.5 (d, C-4), 78.0 (d, C-5), 62.6 (d, C-6), 131.4 (s, C-1'), 116.3 (d, C-2'), 146.0 (s, C-3'), 144.6 (s, C-4'), 117.1 (d, C -5'), 121.2 (d, C-6'), 36.6 (t, C-7'), 72.1 (t, C-8').
[0034] Compound 2: brown solid. UV (MeOH) lambda max (log ...
Embodiment 3
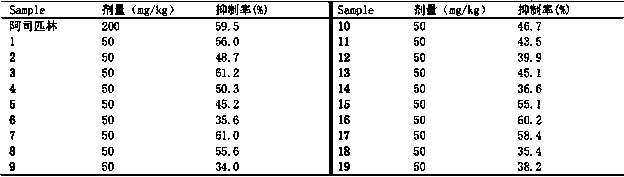
[0053] The analgesic activity detection of compound of the present invention:
[0054] The analgesic activity of the extract of the present invention was determined by acetic acid writhing test. Kunming mice were half male and half male, weighing 18-22 g, fasted and watered 12 hours before the experiment, and randomly divided into control group, sample group, and positive drug group, with 6 mice in each group. The dose of 200 mg / kg was intragastrically administered, the negative control group was given the same amount of normal saline, and the positive drug group was given aspirin. After 40 min of administration, each mouse was intraperitoneally injected with 0.6% glacial acetic acid solution 0.2 mL / 10g. After 3-5 min of glacial acetic acid, observe and record the number of writhing of the mice within 15 min, and calculate the inhibition rate of the test substance on the mouse acetic acid writhing according to the following formula, so as to evaluate the analgesic effect of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com