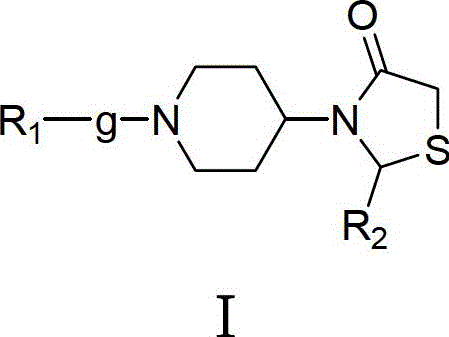
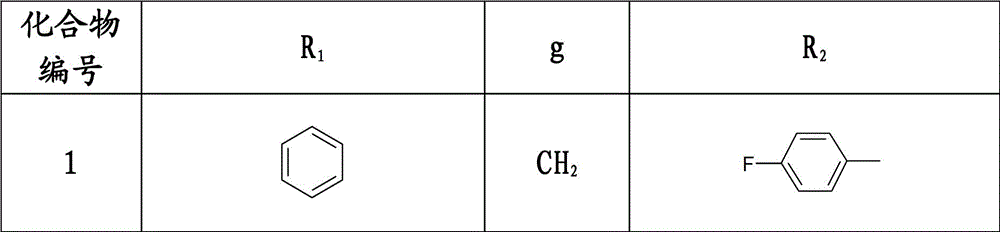
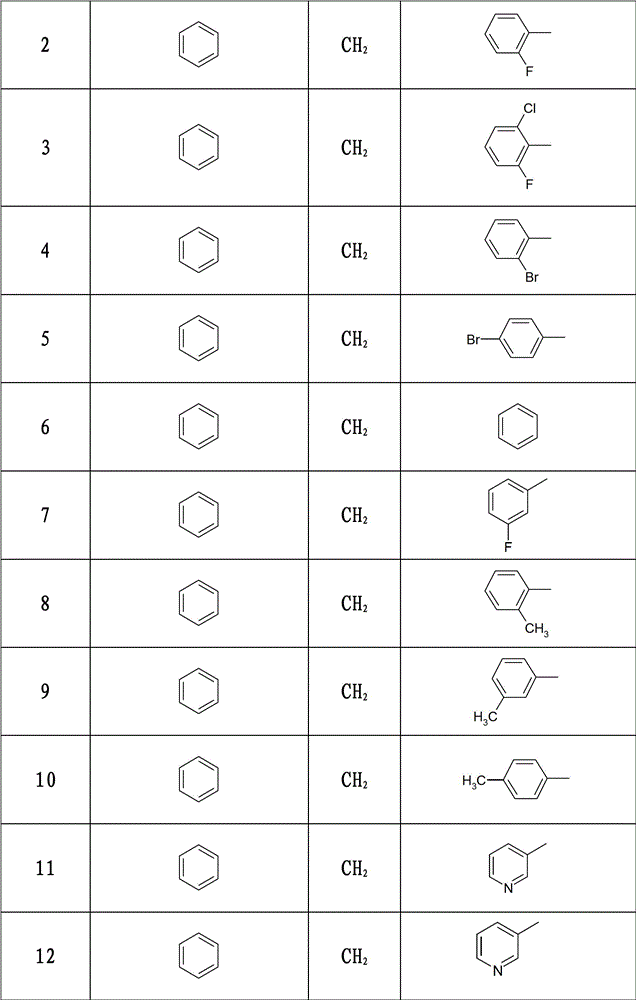
3-(1-arylpiperidin-4-yl)-2-arylthiazolin-4-one compounds, their preparation method and use
A compound, thiazoline technology, applied in the field of medicine and chemical industry, can solve problems such as poor pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1: (±) 3-(1-benzylpiperidin-4-yl)-2-(4-fluorophenyl)-thiazolin-4-one
[0127] Under the protection of nitrogen, dissolve 3.72g (0.03mol) of 4-fluorobenzaldehyde and 5.71g (0.03mol) of 4-amino-1-benzylpiperidine in 50ml of benzene, and the reaction bottle is equipped with a water separator, and the reaction is carried out under reflux for 2 hours , no water came out, lowered to room temperature, added 5.53g (0.06mol) of mercaptoacetic acid, then heated up and refluxed for 2 hours, and anhydrous was generated. Stop the reaction, pour the reaction solution into 30ml of saturated aqueous sodium carbonate solution after being cooled to room temperature, extract with dichloromethane, wash the organic layer with saturated aqueous sodium chloride solution, wash with water, dry over anhydrous sodium sulfate, filter out sodium sulfate, reclaim the dichloromethane, A light yellow oil was obtained, which was recrystallized from absolute ethanol to obtain 10.37 g of a white...
Embodiment 2
[0128] Example 2: (±) 3-(1-benzylpiperidin-4-yl)-2-(2-fluorophenyl)-thiazolin-4-one
[0129] Weigh 2.48g (0.02mol) 2-fluorobenzaldehyde and 3.81g (0.02mol) 4-amino-1-benzylpiperidine, and 3.68g (0.04mol) mercaptoacetic acid, synthesized according to the synthetic method of Example 1, 5.52 g of white solid was obtained, m.p.159-161°C, yield 74.5%. 1 H-NMR (DMSO-d 6 , ppm) δ: 7.05-7.33 (m, 9H), 5.92 (s, 1H), 3.97-4.03 (m, 1H), 3.87-3.91 (dd, 1H, J 1 =1.40Hz,J 2 =15.40Hz), 3.54-3.58(d, 1H, J=15.40Hz), 3.43(s, 2H), 2.72-2.95(m, 2H), 1.91-2.05(m, 3H), 1.45-1.74(m, 3H). MS[M+H] + : 371.2.
Embodiment 3
[0130] Example 3: (±) 3-(1-benzylpiperidin-4-yl)-2-(2-chloro-6-fluorophenyl)-thiazolin-4-one
[0131] Take by weighing 4.77g (0.03mol) 2-chloro-6-fluorobenzaldehyde and 5.71g (0.03mol) 4-amino-1-benzylpiperidine, and 5.53g (0.06mol) mercaptoacetic acid, according to Example 1 Synthetic method Synthesized to obtain 9.12g of white solid, m.p.148-150℃, yield 75%. 1 H-NMR (DMSO-d 6 , ppm) δ: 7.22-7.27 (m, 8H), 6.30 (s, 1H), 3.93-4.02 (m, 2H), 3.58-3.63 (dd, 1H, J 1 =3.92Hz,J 2 =15.40Hz), 3.44(s, 2H), 2.70-2.95(m, 2H), 1.87-2.09(m, 3H), 1.12-1.73(m, 3H). MS[M+H] + : 405.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com