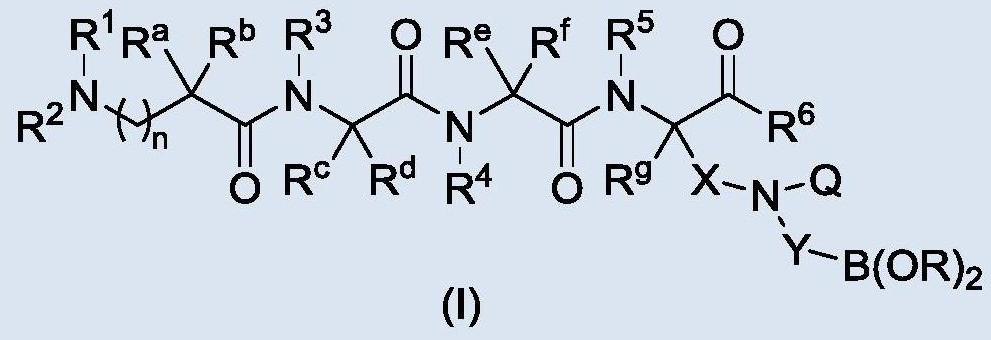
A kind of polypeptide derivative and its use in the field of medicine
A technology of derivatives and polypeptides, which is applied in polypeptide derivatives and its pharmaceutical and pharmaceutical fields to achieve good analgesic activity and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
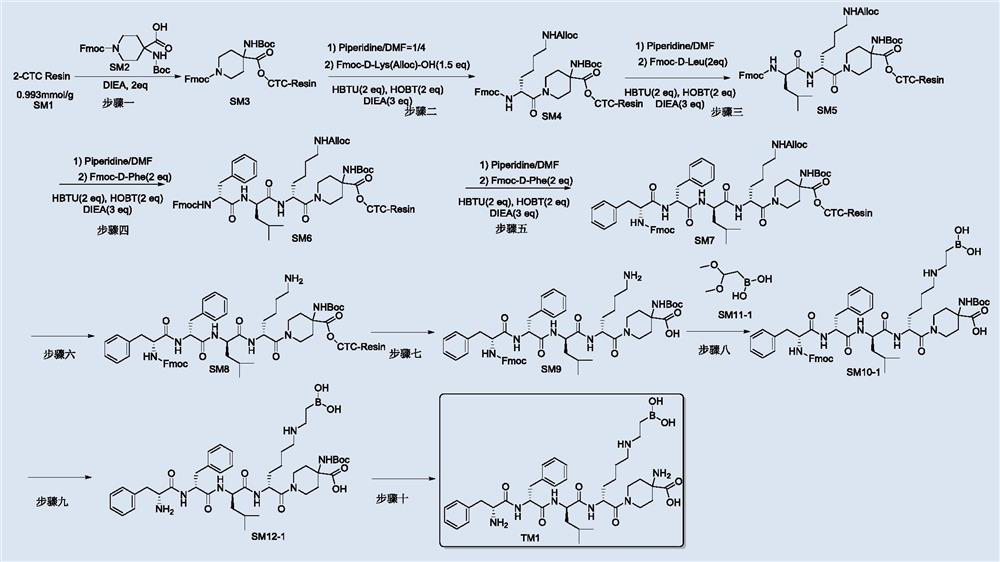
[0076] Such as figure 2 Shown, TM1 can be prepared by the following process steps:
[0077] Step 1: Synthesis of intermediate SM3
[0078] Swell 2-CTC Resin (the degree of substitution is 0.993mmol / g, 2g) with DCM (20mL) at room temperature, the swelling time is 15min, the solvent is removed, SM2 (1.120g, 2.4mmol) and DIEA (0.516g, 4.0 mmol) in DCM (15mL) was added to the swollen resin and reacted for 2h at room temperature; then methanol (2mL) and DIEA (1mL) were added and the reaction was continued for 0.5h; the solvent was drained and washed three times with DCM (30mL) , and finally washed three times with DMF (30mL), and directly put the resin to the next step for reaction.
[0079] Step 2: Synthesis of intermediate SM4
[0080] Add piperidine / DMF (V / V=1 / 4, 20mL) to the product obtained in step 1, react at room temperature for 10min, drain, add piperidine / DMF again (V / V=1 / 4, 20mL ), drained after reacting 10mim at room temperature, and washed 5 times with DMF (30mL), ...
Embodiment 2
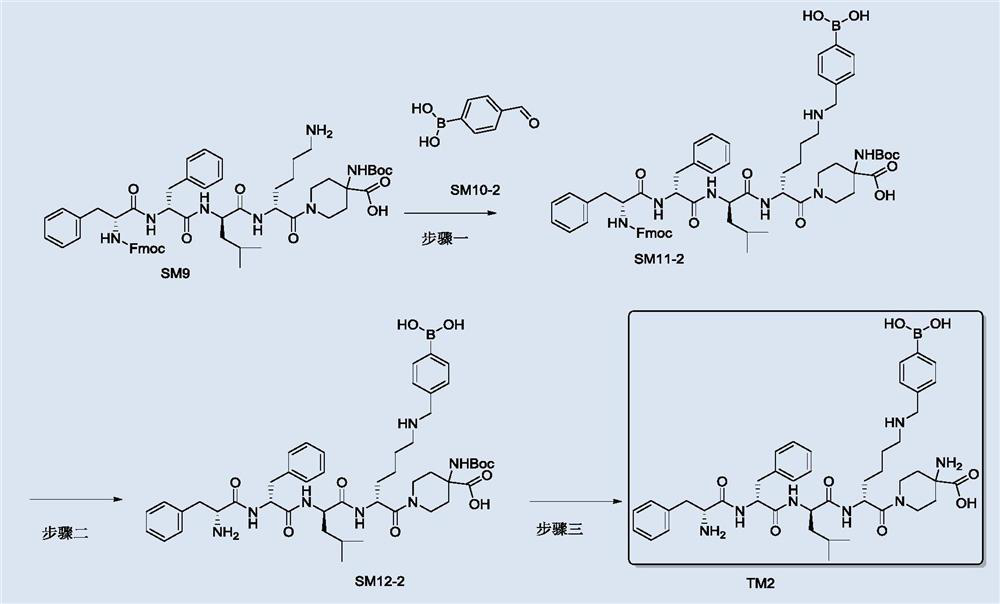
[0102] like image 3 Shown, TM2 can be made through the following process steps:
[0103] Step 1: Synthesis of intermediate SM11-2
[0104] At room temperature, acetic acid (72mg, 0.6mmol), SM9 (300mg, 0.3mmol) and SM10-2 (46mg, 0.3mmol) were dissolved in 1,2-dichloroethane (5mL) for 30min at room temperature, and then Sodium triacetoxyborohydride (126 mg, 0.6 mmol) was added to react at room temperature for 4 h; quenched with 30 mL of water, extracted 3 times with DCM (30 mL), dried the organic phase and spin-dried to obtain the crude product, which was purified by preparative HPLC to obtain Intermediate SM11-2 (211 mg).
[0105] ESI-MS(m / z):1136.6(M+H+)
[0106] Step 2: Synthesis of intermediate SM12-2
[0107] At room temperature, SM11-2 (190 mg, 0.18 mmol) was added to 10 mL of methanol solution of methylamine, stirred and reacted for 1 h at room temperature, and the reaction solution was directly spin-dried, and the obtained crude product was purified by preparative H...
Embodiment 3
[0114] like Figure 4 Shown, TM3 can be prepared by the following process steps:
[0115] Step 1: Synthesis of intermediate SM11-3
[0116] At room temperature, acetic acid (72mg, 0.6mmol), SM9 (300mg, 0.3mmol) and SM10-3 (45mg, 0.3mmol) were dissolved in 1,2-dichloroethane (5mL) for 30min at room temperature, and then Sodium triacetoxyborohydride (126 mg, 0.6 mmol) was added to react at room temperature for 4 h; quenched with 30 mL of water, extracted 3 times with DCM (30 mL), dried the organic phase and spin-dried to obtain the crude product, which was purified by preparative HPLC to obtain Intermediate SM11-3 (205 mg).
[0117] ESI-MS(m / z):1136.6(M+H+)
[0118] Step 2: Synthesis of intermediate SM12-3
[0119] At room temperature, SM11-3 (190 mg, 0.18 mmol) was added to 10 mL of methanol solution of methylamine, stirred and reacted at room temperature for 1 h, and the reaction solution was directly spin-dried to obtain the crude product, which was purified by preparativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com