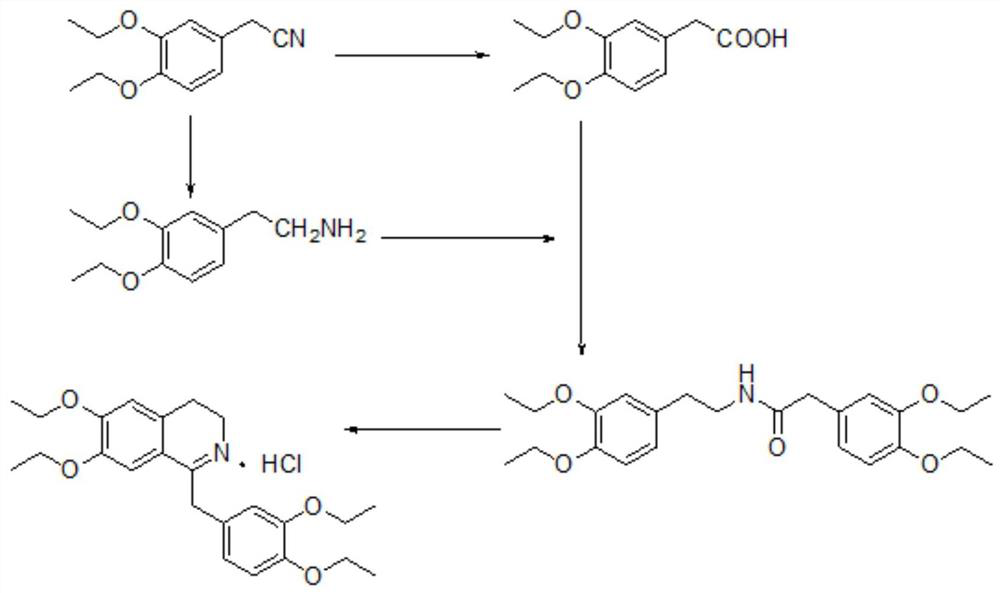
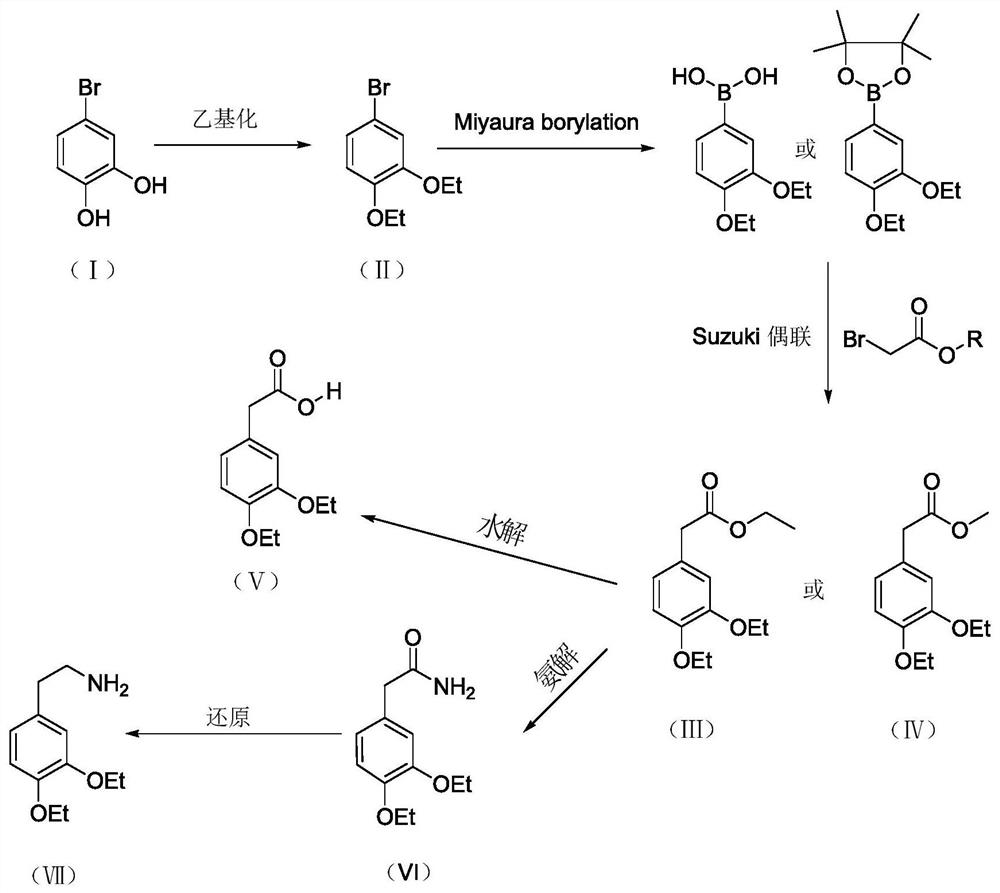
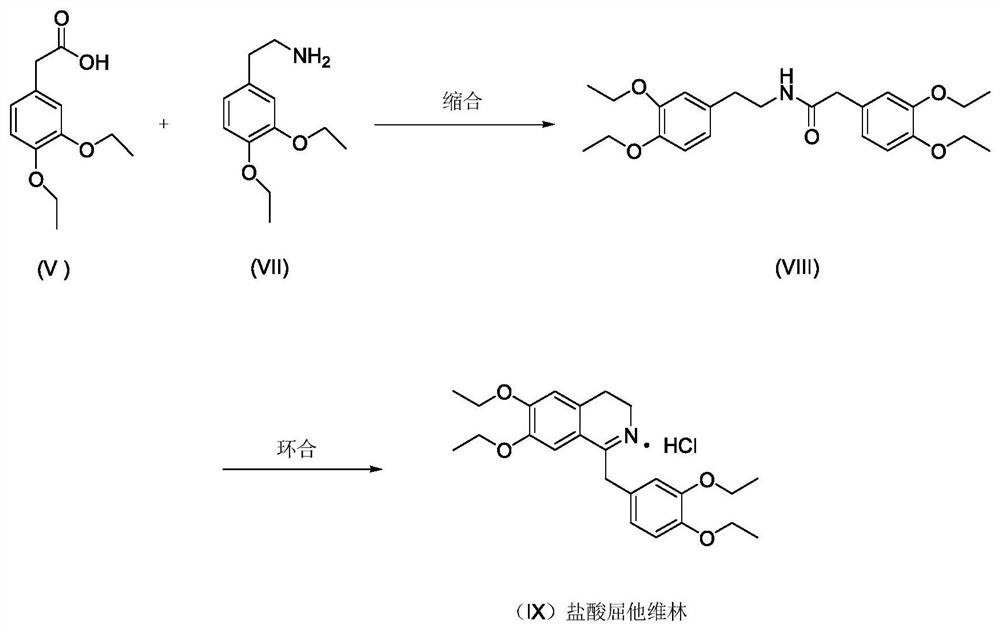
A kind of preparation new method of drotaverine hydrochloride intermediate
A technology for drotaverine hydrochloride and intermediates, which is applied in the field of preparing drotaverine hydrochloride intermediates, can solve the problems of high equipment requirements, operator safety, environmental protection risks, potential safety hazards, and the like, and achieves safe and efficient solutions. Environmental concerns, avoidance of reaction steps, good practical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of preparation new method of drotaverine hydrochloride intermediate 3,4-diethoxyphenylacetic acid (V), comprises the following steps:
[0036] 1) Synthesis of 1,2-diethoxy-4-bromobenzene (II)
[0037] Add 529 mmol of 4-bromocatechol (I) and 3 L of acetonitrile into the four-neck flask, add 2.113 mol of potassium carbonate and 1.693 mol of iodoethane under stirring conditions, raise the temperature to 70-80°C, and keep the temperature for 4-6 hours. After the reaction was completed, filter at room temperature, evaporate the filtrate to dryness under reduced pressure, add 600 mL of ethyl acetate to dissolve it, and wash with water 3 times, 100 mL each time. Dry over anhydrous sodium sulfate and evaporate to dryness under reduced pressure to obtain a yellow oily substance, namely 449 mmol of 1,2-diethoxy-4-bromobenzene (II), with a molar yield of 84.9%. Product 1,2-diethoxy-4-bromobenzene (II) is characterized, 1 H NMR (500MHz, MeOD): δ: 7.033~7.038(s, 1H, -ArH), ...
Embodiment 2
[0043] A kind of preparation new method of drotaverine hydrochloride intermediate 3,4-diethoxyphenethylamine (VII), comprises the following steps:
[0044] 1) Synthesis of 1,2-diethoxy-4-bromobenzene (II)
[0045] Add 529 mmol of 4-bromocatechol (I) and 3 L of acetonitrile into the four-neck flask, add 2.113 mol of potassium carbonate and 1.693 mol of iodoethane under stirring conditions, raise the temperature to 70-80°C, and keep the temperature for 4-6 hours. After the reaction was completed, filter at room temperature, evaporate the filtrate to dryness under reduced pressure, add 600 mL of ethyl acetate to dissolve it, and wash with water 3 times, 100 mL each time. Dry over anhydrous sodium sulfate and evaporate to dryness under reduced pressure to obtain a yellow oily substance, namely 449 mmol of 1,2-diethoxy-4-bromobenzene (II), with a molar yield of 84.9%. Product 1,2-diethoxy-4-bromobenzene (II) is characterized, 1 H NMR (500MHz, MeOD): δ: 7.033~7.038(s, 1H, -ArH), 6...
Embodiment 3
[0053] A kind of preparation new method of drotaverine hydrochloride intermediate 3,4-diethoxyphenylacetic acid (V), comprises the following steps:
[0054] 1) Synthesis of 1,2-diethoxy-4-bromobenzene (II)
[0055] Add 529 mmol of 4-bromocatechol (I) and 3 L of acetonitrile into a four-neck flask, add 2.138 mol of potassium hydroxide and 1.167 mol of diethyl sulfate under stirring conditions, raise the temperature to 70-80°C, and keep the temperature for 4-6 hours. After the reaction was completed, filter at room temperature, evaporate the filtrate to dryness under reduced pressure, add 600 mL of ethyl acetate to dissolve, wash with 5% sodium hydroxide solution 3 times, 100 mL each time, and wash 3 times with water, 100 mL each time. Dry over anhydrous sodium sulfate and evaporate to dryness under reduced pressure to obtain a yellow oily substance, namely 457 mmol of 1,2-diethoxy-4-bromobenzene (II), with a molar yield of 86.4%. Characterize the product 1,2-diethoxy-4-bromobe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com