A kind of preparation method of sapropterin hydrochloride
A technology of sapropterin hydrochloride and reaction, which is applied in the preparation of hydrazone, preparation of sugar derivatives, chemical instruments and methods, etc., can solve the problems of complicated operation and low yield, achieve high purity, high yield, and reduce process Effect with process time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
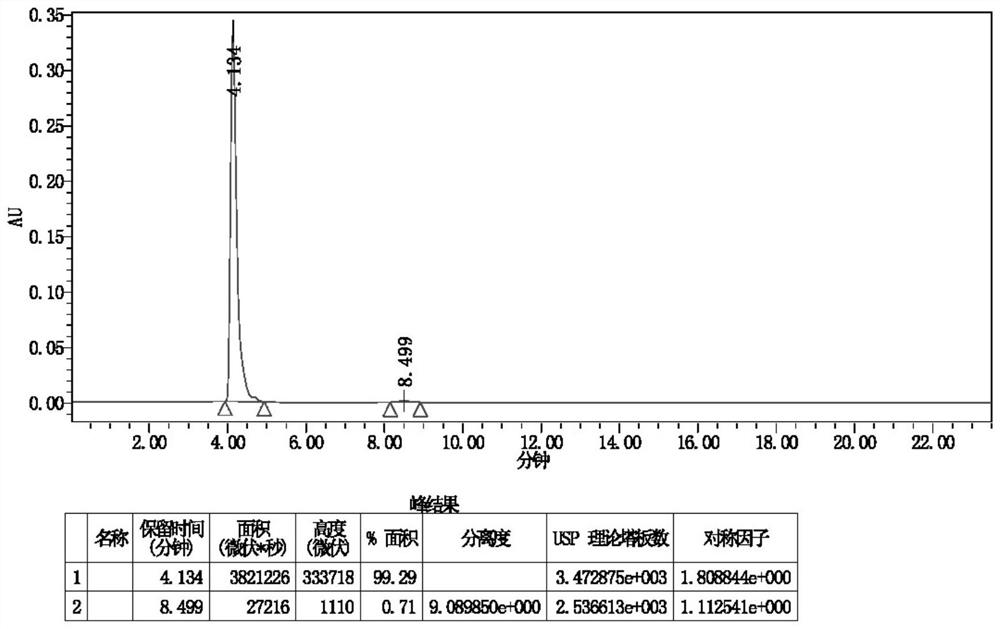
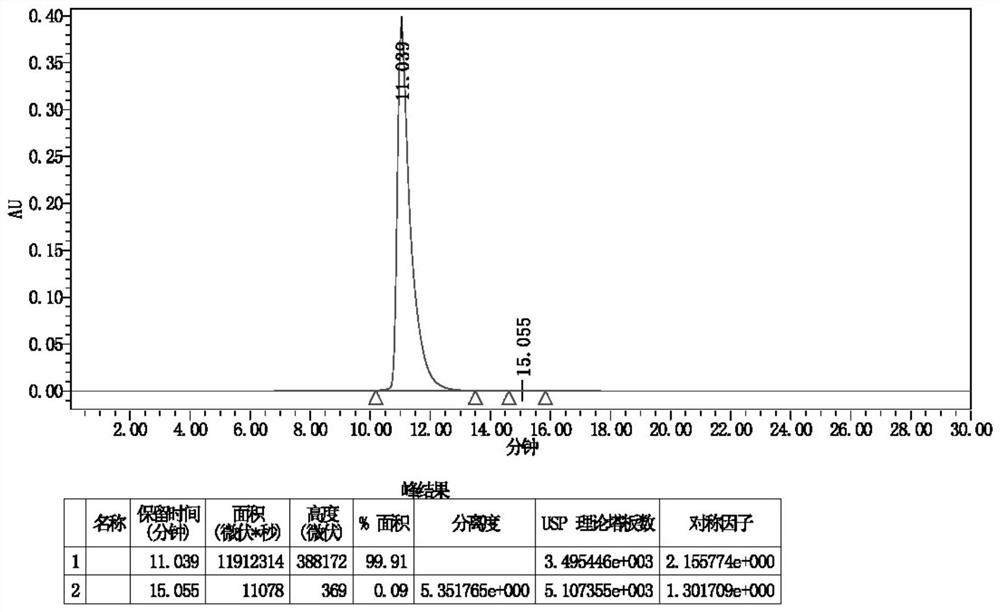
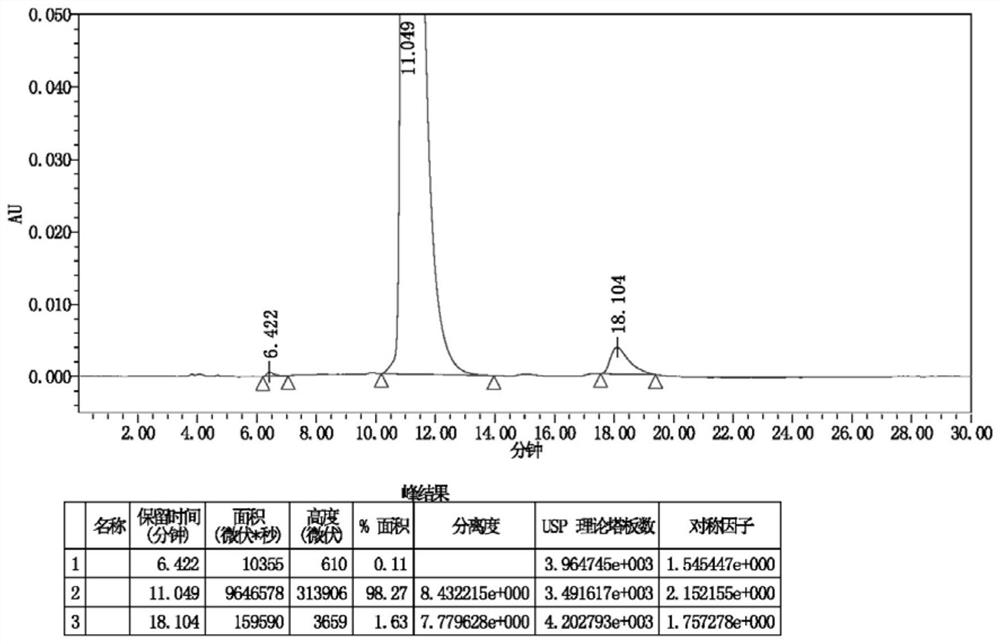
Image
Examples
example 1
[0037] The synthesis of example 1 DL-1:
[0038] Add DL-SMA (134g, 1.0mol), ethyl acetate 1.0L, 4-dimethylaminopyridine 24.4g to the reaction vessel in sequence, cool down to 10~15°C, add acetic anhydride (326.7g, 3.2mol) dropwise, Control the internal temperature at 15-20°C, after dropping, control the reaction temperature at 0-20°C, monitor by TLC until the reaction is complete. Post-treatment: Quench with saturated aqueous sodium carbonate solution, collect the organic phase after separation, extract the aqueous phase with ethyl acetate (500mL*2), combine the organic phases, and wash with saturated aqueous sodium carbonate solution (400mL*1), collect the organic phase The phase was spin-dried, and the ethyl acetate was recovered to obtain a light yellow oil DL-1 (260 g), which was directly carried out to the next reaction.
example 2
[0039] The synthesis of example 2 DL-2:
[0040] Add DL-1 (260g) and 900mL water obtained in the previous reaction to the reaction vessel, add dropwise acetic acid to adjust the pH value to 3-7, then add phenylhydrazine (118.8g, 1.1mol, 1.1eq), stir and React overnight at room temperature under protection. TLC monitoring, after the reaction was completed, the reaction solution was extracted twice with ethyl acetate (500mL*2), the organic phases were combined, dried over anhydrous sodium sulfate, filtered to remove the desiccant, and spin-dried to obtain a yellow oil DL-2 (340g). Proceed directly to the next reaction.
example 3
[0041] The synthetic method 1 of example 3 DL-4:
[0042] Add DL-2 (181g, 0.51mol) and 600mL of methanol into the reaction vessel, and stir until completely dissolved. Anhydrous lithium perchlorate (1.8 g, 0.187 mol) was added, and the reaction was stirred for 30 minutes. Under temperature control at 30±5°C, add DL-SMB (151 g, 0.63 mol) aqueous sodium hydroxide solution prepared in advance. Under the protection of nitrogen, the temperature was raised to reflux, and the reaction was carried out overnight, and monitored by TLC until the end of the reaction. Cool down to 10±5°C, add dropwise a solution of iodine (328g, 1.29mol) in methanol (600mL), and react at room temperature until the reaction is completed under TLC monitoring. Filter, rinse with methanol, and then wash with water, and dry the obtained solid at 50°C to constant weight to obtain light brown solid DL-4 (300g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com