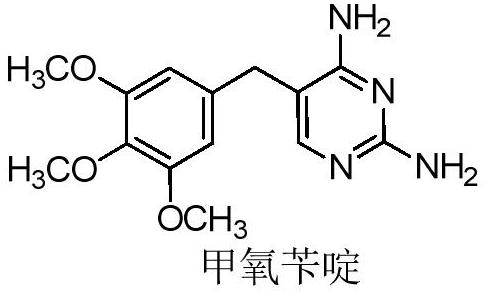
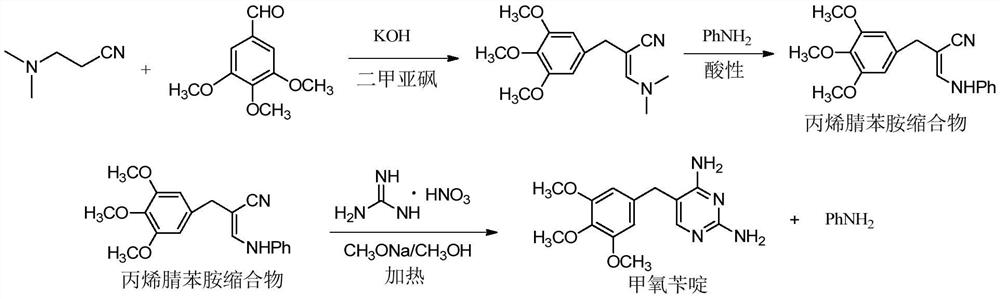
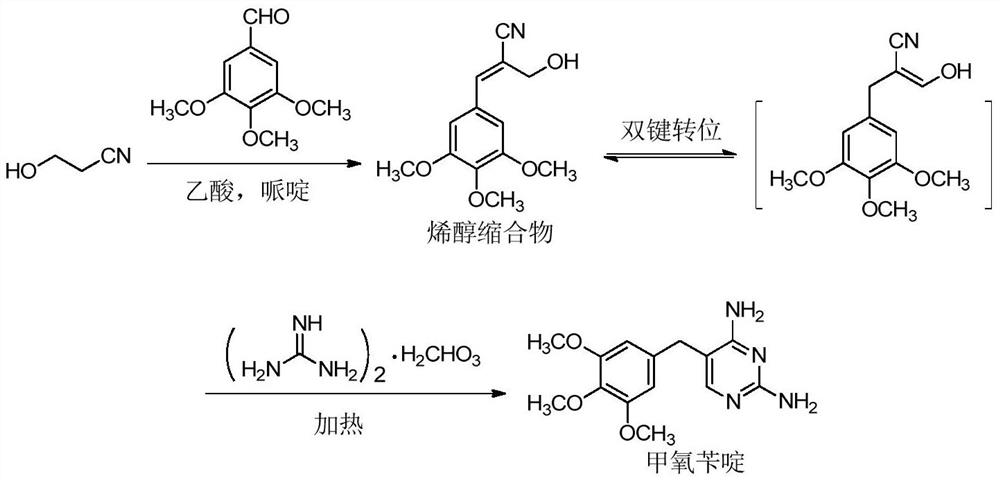
Preparation method of trimethoprim
A technology of trimethoprim and trimethoxybenzaldehyde, which is applied in the field of high-yield preparation of raw material drug trimethoprim, can solve the problem of extremely strict quality control of trimethoprim, high energy consumption of recovery auxiliary raw materials, nitric acid Guanidine has problems such as safety risks, and achieves the effects of improving labor protection and safety production, facilitating large-scale industrial production, and rationally designing synthetic processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] In a three-necked flask equipped with mechanical stirring, 750 mL of glacial acetic acid, 3-hydroxypropionitrile (78.2 g, 1.10 mol) and catalyst piperidine (8.5 g, 0.10 mol) were added in turn, and stirred evenly; 3,4,5- Trimethoxybenzaldehyde (196.2 g, 1.0 mol) was dissolved by stirring, and stirred vigorously at room temperature overnight to complete the Knoevenagel condensation reaction. Then, guanidine carbonate solid (103.6 g, 0.575 mol) was slowly added in batches at room temperature, stirred to dissolve, and carbon dioxide gas was slowly released. After adding guanidine carbonate, stirring was continued at room temperature for 0.5 h. The temperature was raised to 55°C, and the cyclization reaction was completed by stirring continuously for 5h at this temperature. After the reaction was completed, the acetic acid in the reaction system was evaporated under reduced pressure, 800 mL of deionized water was added and the temperature was raised to 60 ° C an...
Embodiment 2
[0034] In a three-necked flask equipped with mechanical stirring, 800 mL of glacial acetic acid, 3-hydroxypropionitrile (81.7 g, 1.15 mol) and catalyst piperidine (12.8 g, 0.15 mol) were sequentially added, and the mixture was stirred uniformly. Add 3,4,5-trimethoxybenzaldehyde (196.2 g, 1.0 mol), stir to dissolve, and stir vigorously at room temperature for 10 h to complete the Knoevenagel condensation reaction. Then, guanidine carbonate solid (108.1 g, 0.60 mol) was slowly added in batches at room temperature, stirred to dissolve, and carbon dioxide gas was slowly released. After adding guanidine carbonate, stirring was continued at room temperature for 0.5 h. The temperature was raised to 60 °C, and the cyclization reaction was completed at this temperature with continuous stirring for 5 h. After the reaction was completed, the acetic acid in the reaction system was evaporated under reduced pressure, 800 mL of deionized water was added and the temperature was raised to 60 °...
Embodiment 3
[0036] In a three-necked flask equipped with mechanical stirring, 850 mL of glacial acetic acid, 3-hydroxypropionitrile (81.7 g, 1.15 mol) and catalyst piperidine (17.0 g, 0.2 mol) were sequentially added, and the mixture was stirred uniformly. Add 3,4,5-trimethoxybenzaldehyde (196.2 g, 1.0 mol), stir to dissolve, and stir vigorously at room temperature for 8 h to complete the Knoevenagel condensation reaction. Then, guanidine carbonate solid (108.1 g, 0.60 mol) was slowly added in batches at room temperature, stirred to dissolve, and carbon dioxide gas was slowly released. After adding guanidine carbonate, stirring was continued at room temperature for 0.5 h. The temperature was raised to 50 °C, and the cyclization reaction was completed by stirring continuously for 6 h at this temperature. After the reaction was completed, the acetic acid in the reaction system was evaporated under reduced pressure, 750 mL of deionized water was added and the temperature was raised to 65°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com