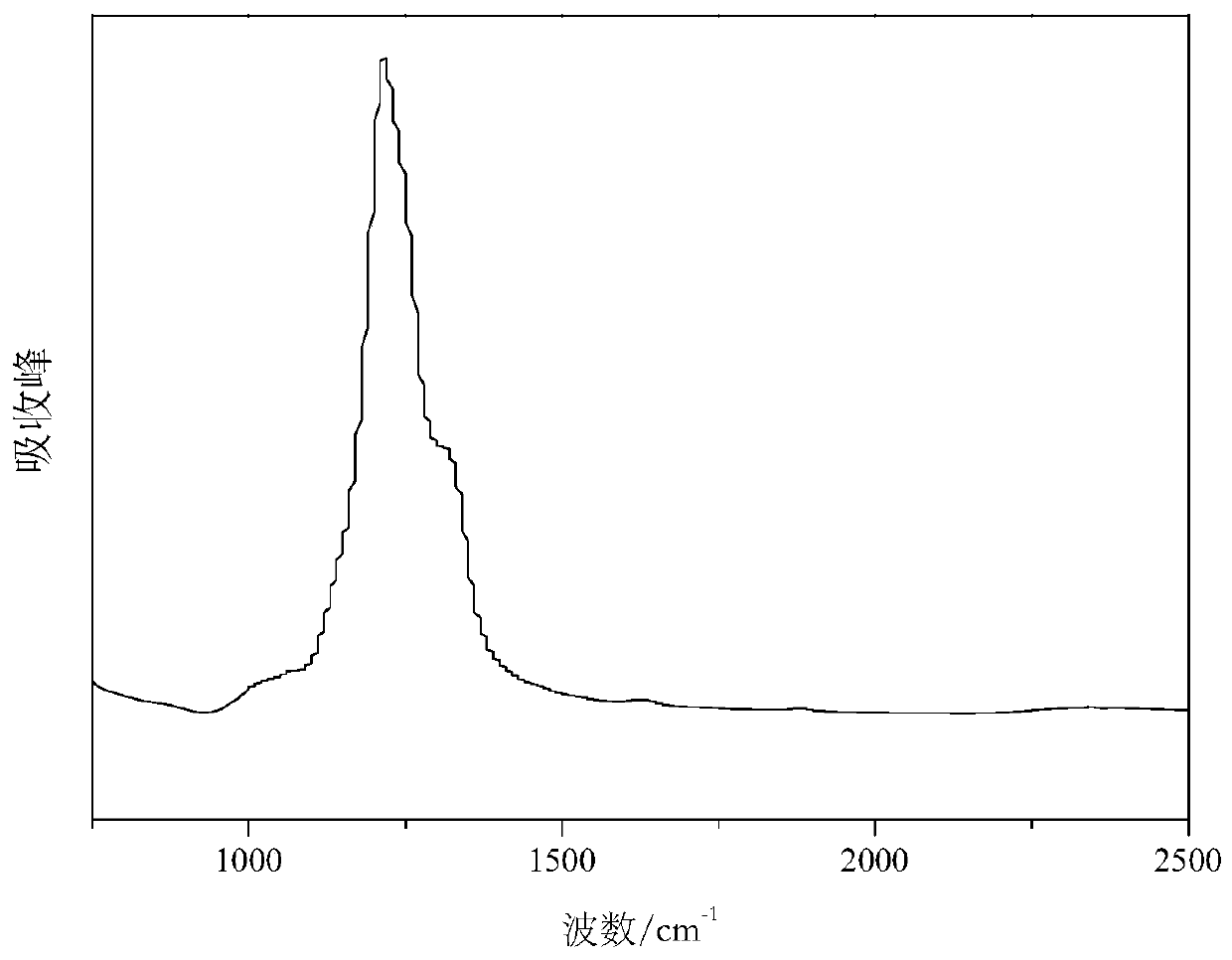
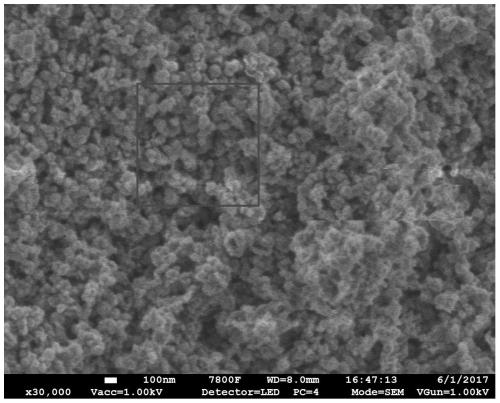
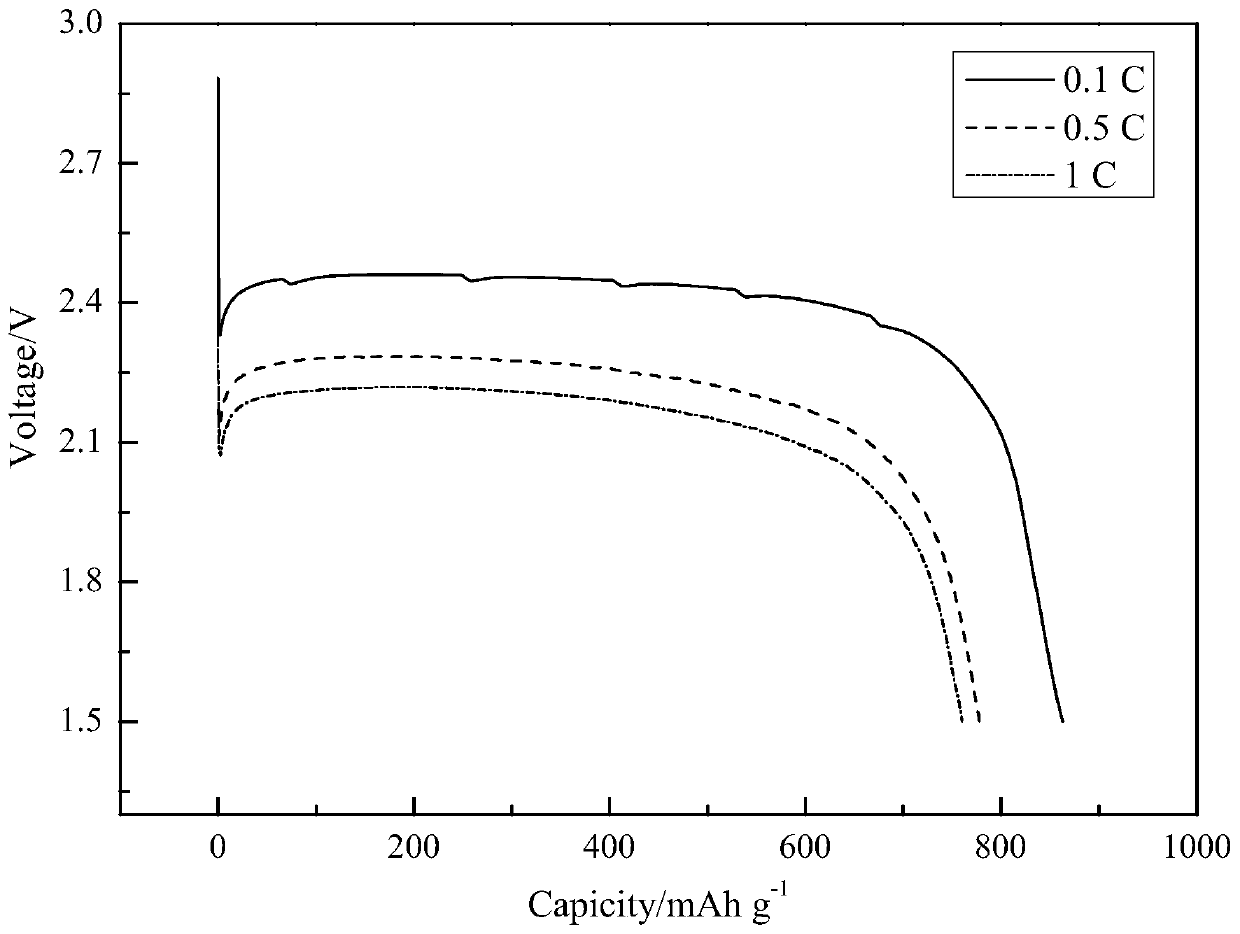
Method for preparing carbon fluorine material
A technology of carbon fluoride and carbon materials, applied in the direction of carbon fluoride, structural parts, electrical components, etc., can solve the problems of high temperature, long fluorination time, etc., to reduce the preparation cost, short fluorination time, and good electrochemical performance. performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) 1g of petroleum coke is put into the reaction vessel, slowly feed nitrogen into the reaction vessel through the air inlet to make its internal pressure reach 0.1MPa, after holding the pressure for 24h, the pressure variation inside the reaction vessel is less than 5%, then The pressure of the reaction vessel is qualified, and the gas outlet of the reaction vessel is slowly opened to release the pressure so that the internal pressure drops to normal pressure;
[0030] (2) The air inlet of the reaction vessel is connected with the reaction gas source, and the gas outlet of the reaction vessel is connected with the container containing the aqueous sodium hydroxide solution; then, the temperature in the reaction vessel is controlled at 400° C., and the Fill the reaction gas, and the pressure in the reaction vessel is normal pressure. After continuously filling the reaction gas for 6 hours, the reaction will form CF x ; Wherein, the reaction gas is a mixed gas composed o...
Embodiment 2
[0036] (1) 1g of petroleum coke is put into the reaction vessel, slowly feed nitrogen into the reaction vessel through the air inlet to make its internal pressure reach 0.2MPa, after holding the pressure for 18h, the pressure variation inside the reaction vessel is less than 5%, then The pressure of the reaction vessel is qualified, and the gas outlet of the reaction vessel is slowly opened to release the pressure so that the internal pressure drops to normal pressure;
[0037] (2) The air inlet of the reaction vessel is connected with the reaction gas source, and the gas outlet of the reaction vessel is connected with the container containing the aqueous sodium hydroxide solution; then, the temperature in the reaction vessel is controlled at 380° C., and the Fill the reaction gas, and the pressure in the reaction vessel is normal pressure. After continuously filling the reaction gas for 8 hours, the reaction will form CF x ; Wherein, the reaction gas is a mixed gas composed o...
Embodiment 3
[0043](1) 1g of petroleum coke is put into the reaction vessel, slowly feed argon into the reaction vessel through the air inlet to make its internal pressure reach 0.2MPa, after holding the pressure for 15h, the pressure variation inside the reaction vessel is less than 5%, Then the pressure-holding of the reaction vessel is qualified, and the gas outlet of the reaction vessel is slowly opened to release the pressure so that the internal pressure is reduced to normal pressure;
[0044] (2) The air inlet of the reaction vessel is connected with the reaction gas source, and the gas outlet of the reaction vessel is connected with the container filled with potassium hydroxide aqueous solution; then, the temperature in the reaction vessel is controlled at 350° C. The reaction gas is filled in the medium, and the pressure in the reaction vessel is normal pressure. After continuously filling the reaction gas for 6 hours, the reaction forms CF x ; Wherein, the reaction gas is a mixed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com