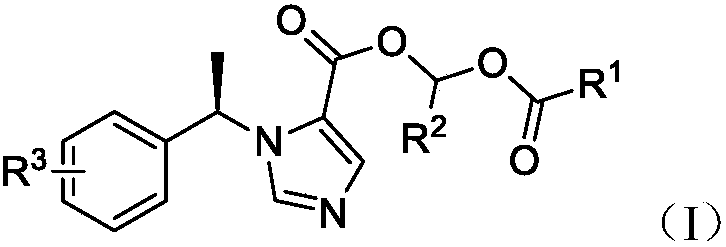
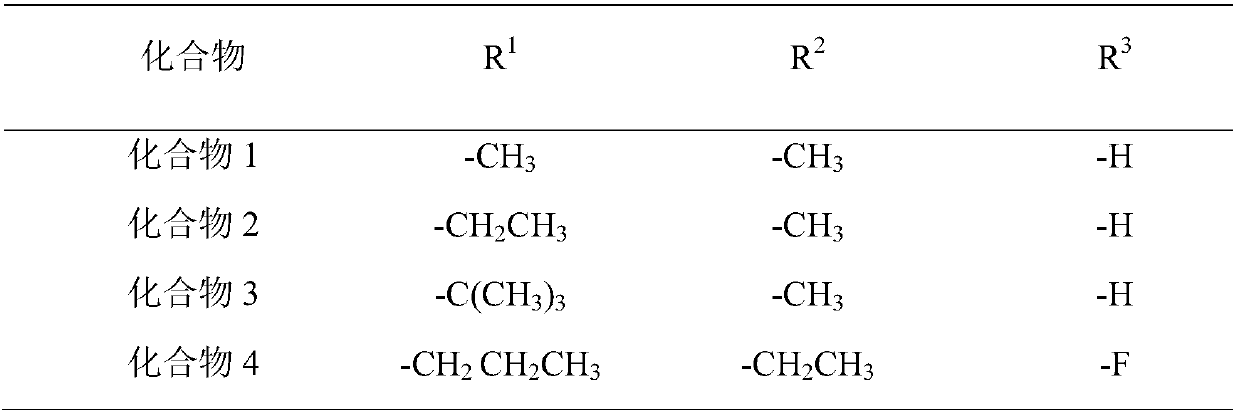
Etomidate derivative and preparation method thereof
A compound, methyl technology, applied in the field of anesthetic drugs, can solve the problems of increased infection, unfavorable recovery of patients, and reduced usage rate of etomidate, etc., and achieves low toxicity and side effects, good prospects, and short maintenance time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] 1.1 Synthesis of Compound M
[0039] Etomidate (10.1g, 0.04mol) was dissolved in 100ml of methanol, and an aqueous solution of LiOH (2.0g, 0.08mol, 2eq, dissolved in 20ml of water) was added, then the reaction solution was stirred at room temperature for 5 hours, and then spun under reduced pressure. Dry methanol, adjust the pH of the aqueous phase to 2-3 with 1N HCl, and extract twice with ethyl acetate, combine the organic phases, and dry over anhydrous magnesium sulfate. Spin-dry under reduced pressure to obtain 7.1 g of white solid, yield 79%
[0040] LCMS:218[M+H] +
[0041] 1 H-NMR (DMSO, 300MHz)δ11.60(bs,1H),δ7.96(s,1H),δ7.84(s,1H),δ6.93-6.77(m,5H),δ5.15( bs, 1H), δ1.92 (d, 3H).
[0042] 1.2 Synthesis of compound 1
[0043] Dissolve M (1g, 4.6mmol, 1eq) in 15ml of acetonitrile, add K 2 CO 3 (1.3g, 9.2mmol, 2eq), the reaction solution was cooled to 0°C, and ethyl 1-bromoacetate (1.2g, 7.1mmol, 1.5eq) was added, and the reaction solution was slo...
Embodiment 2
[0047] Compound 2 can be prepared by the following reaction scheme
[0048]
[0049] Compound M (1.1g, 5mmol) was dissolved in dichloromethane solution (10ml), potassium carbonate (1.4g, 10mmol) was added to react at room temperature for 2 hours, cooled to 0-5°C, and 1-bromoethyl propionate was added (1.8g, 10mmol) Adjust the temperature to 10-15°C and stir the reaction for 30 hours, filter, remove the solvent in a rotary evaporator, and purify by column chromatography (ethyl acetate:n-hexane=1:4) to obtain light yellow 0.5 g of solid, yield 31%.
[0050] LCMS:317([M+H]+).
[0051] 1 H-NMR (CDCl 3 ,300MHz)δ7.88(s,1H),δ7.86(s,1H),δ7.25-7.05(m,5H),δ6.62(m,2H),δ5.17(m,1H), δ2.31(m,2H), δ1.89(d,3H), δ1.75(m,3H), δ1.15(m,3H)
Embodiment 3
[0053] Compound 3 can be prepared by the following reaction scheme
[0054]
[0055] Compound M (1.2g, 5.5mmol) was dissolved in N,N-dimethylformamide (10ml), sodium carbonate (0.6g, 6.6mmol) was added, and reacted at room temperature for 1 hour. Cool down to 0-5°C, add 1-bromoethyl tert-butyl carboxylate (1.3g, 6.0mmol), adjust the temperature to 10-15°C, stir for 36 hours, filter, add 100ml of purified water, add 30ml of ethyl acetate for extraction Three times, the solvent was removed in a rotary evaporator, and purified by column chromatography (ethyl acetate:n-hexane=1:5) to obtain 0.4 g of a light yellow solid with a yield of 21%.
[0056] MS (ESI) m / z 345 ([M+H] + )
[0057] 1 H-NMR (CDCl 3 ,300MHz)δ7.89(s,1H),δ7.86(s,1H),δ7.25-7.05(m,5H),δ6.59(m,1H),δ5.16(m,1H), δ1.89(s, 3H), δ1.72(d, 3H), δ1.24(s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com