Method for establishing recombinant adenovirus vector with Africa swine fever EP153R and P54 gene coexpression and packaging adenovirus
A technology of EP153R, African swine fever virus, applied in the direction of virus/phage, antiviral agent, genetic engineering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The optimized synthesis of embodiment 1 gene
[0039] Query the EP153R gene (GI: 22220439), P54 gene (GI: 290782550) and the CTLA4 gene (NC_010457.5) from the porcine cell line included in the NCBI website, and obtain optimization (referring to optimizing the nucleotide sequence) through artificial synthesis ) after the gene, the sequence is as SEQ ID No.1-3.
[0040] CTLA4 sequence Seq ID No.1
[0041] GCTAGAGATCTGGTACCGCCACCATGCACGTGGCCCAACCTGCAGTA GTGCTGGCCAACAGCCGGGGTGTTGCCAGCTTTGTGTGTGAGTATGGGTC TGCAGGCAAAGCTGCCGAGGTCCGGGTGACAGTGCTGCGGCGGGCCGGC AGCCAGATGACTGAAGTCTGTGCCGCGACATATACTGTGGAGGATGAGTT GACCTTCCTTGATGACTCTACATGCACTGGCACCTCCACCGAAAACAAAG TGAACCTCACCATCCAAGGGCTGAGAGCCGTGGACACTGGGCTCTACATC TGCAAGGTGGAGCTCCTGTACCCACCACCCTACTATGTGGGTATGGGCAA CGGGACCCAGATTTATGTCATTGATCCAGAACCATGCCCAGATTCTGATA GTACTGATTACAAAGACGATGACGATAAG
[0042] EP153R sequence Seq ID No.2
[0043] AAAGACGATGACGATAAGACCGGTTTCAGCAACAAGAAGTACATCG GCCTGATCAACAAGAAGGAGGGCCTGAAGAAGAAGATCGATGACTACA...
Embodiment 2
[0046] Modification and amplification of fragments of embodiment 2
[0047] 1) Primer design, see Table 1:
[0048] Table 1 Primer design
[0049]
[0050] 2) CTLA4, EP153R, P54 fragment PCR
[0051] CTLA4: CTLA4-KpnI-F+CTLA4-R+CTLA4-Flag-R
[0052] EP153R: EP153R-Flag-F+EP153R-Myc-R
[0053] P54: P54-Myc-F+P54-HA-R+HA-R
[0054] CTLA4-EP153R-P54: CTLA4-KpnI-F+EP153R-Myc-R+HA-R (template is CTLA4+EP153R+P54)
[0055] That is, when amplifying the CTLA4 gene (PCR product 1), the primer combination of CTLA4-KpnI-F+CTLA4-R+CTLA4-Flag-R is used, and the DNA template is Seq ID No.1; when amplifying the EP153R gene (PCR product 2) The primer combination of EP153R-Flag-F+EP153R-Myc-R is used, and the DNA template is Seq ID No.2; when amplifying P54 (PCR product 3), the primer combination of P54-Myc-F+P54-HA-R+HA-R is used Primer combination, DNA template is Seq ID No.3. When amplifying CTLA4-EP153R-P54 (PCR product 4), the primer combination of CTLA4-KpnI-F+EP153R-Myc-R+HA-R...
Embodiment 3
[0065] Example 3 Restriction digestion and homologous recombination of pShuttle-CMV vector
[0066] 1) Digest the pShuttle-CMV vector, and the restriction system is shown in Table 4.
[0067] Table 4 enzyme digestion system
[0068] Reaction solution composition
volume
Plasmid (350μg / μL)
20 μL
10×Buffer
5μL
KpnI
2μL
Hind III
2μL
wxya 2 o
31μL
total
50μL
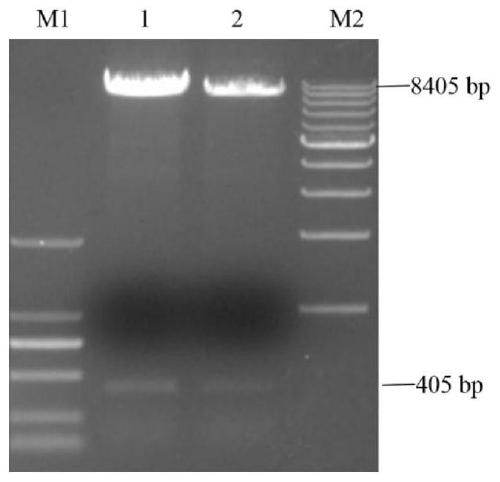
[0069] After adding the sample and mixing it, place it at 37°C for enzyme digestion for 6 hours. After the reaction, use 1% agarose gel electrophoresis to detect the size of the digested band, and use a gel recovery kit to recover the linearized plasmid.
[0070] 2) Vector and fragment homologous recombination:
[0071] Table 5 Reaction system of vector and fragment homologous recombination
[0072]
[0073]
[0074] Wherein CTLA4-EP153-P54 is the aforementioned PCR product 4.
[0075] React at 37°C for 35 minutes, transform DH5α E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com