Application of α-mangostin derivatives in the preparation of anti-cerebral palsy drugs and anti-cerebral palsy drug composition
A technology of mangostin and its derivatives, which is applied in the field of medicine, can solve the problems of unclear exact mechanism, achieve the effect of restoring the ability of learning and memory, improving the ability of gait balance, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
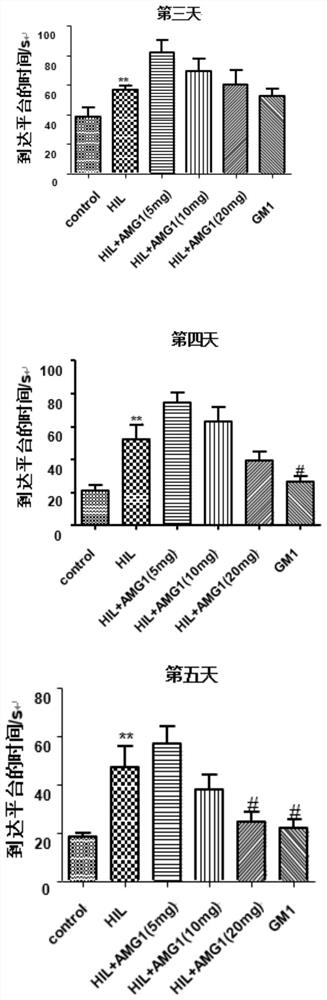
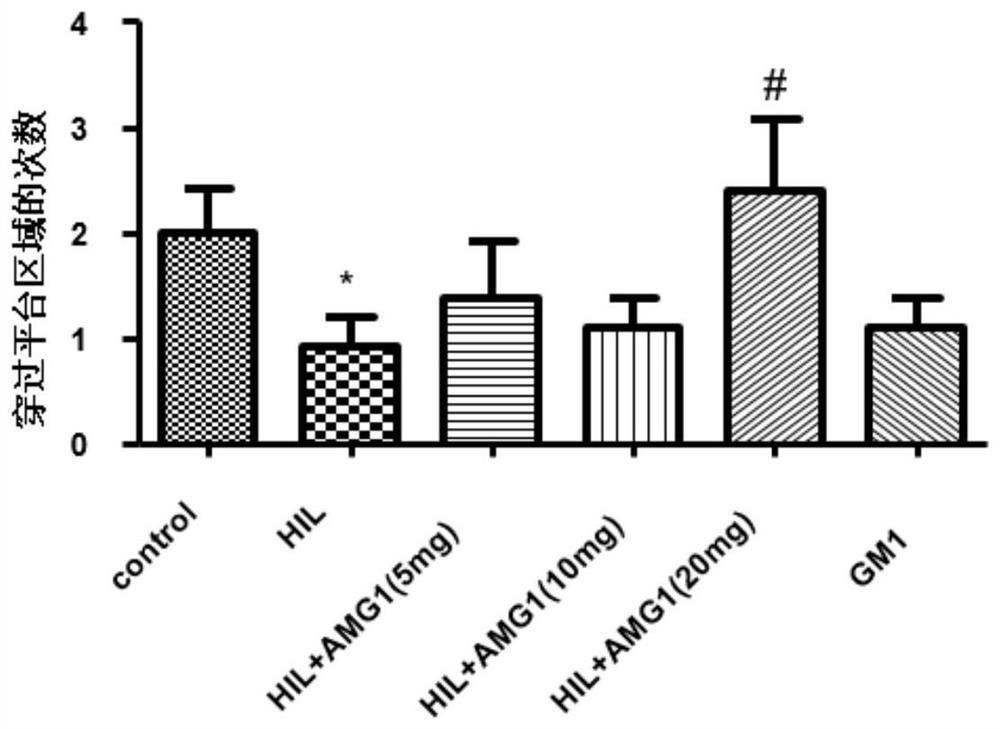
[0035] Effects of α-Mangostin Derivatives Administration on Morris Water Maze Test in Rats with Cerebral Palsy
[0036] 1. Animal groups: SD rats (6 days old, 12-18 g, 15 rats in each group), randomly divided into groups. The grouping is shown in Table 1.1.
[0037] Table 1.1 Animals and groups required in Example 1
[0038]
[0039] 2. Administration and dosage: α-mangostin derivatives with a purity of more than 99% are dissolved in ethyl oleate for injection, and the animals are administered by intraperitoneal injection. The dosage is: 5mg / kg, 10mg / kg, 20mg / kg; Positive drug ganglioside GM1 dose: 20 mg / kg, as a positive control; both the model group and the sham operation group were intraperitoneally injected with the same amount of solvent (ethyl oleate). Administration was administered at 9:00 every day, and the administration was continued for 20 days after modeling.
[0040] 3. Surgical procedure: After the animal is anesthetized, the neck skin is prepared, the nec...
Embodiment 2
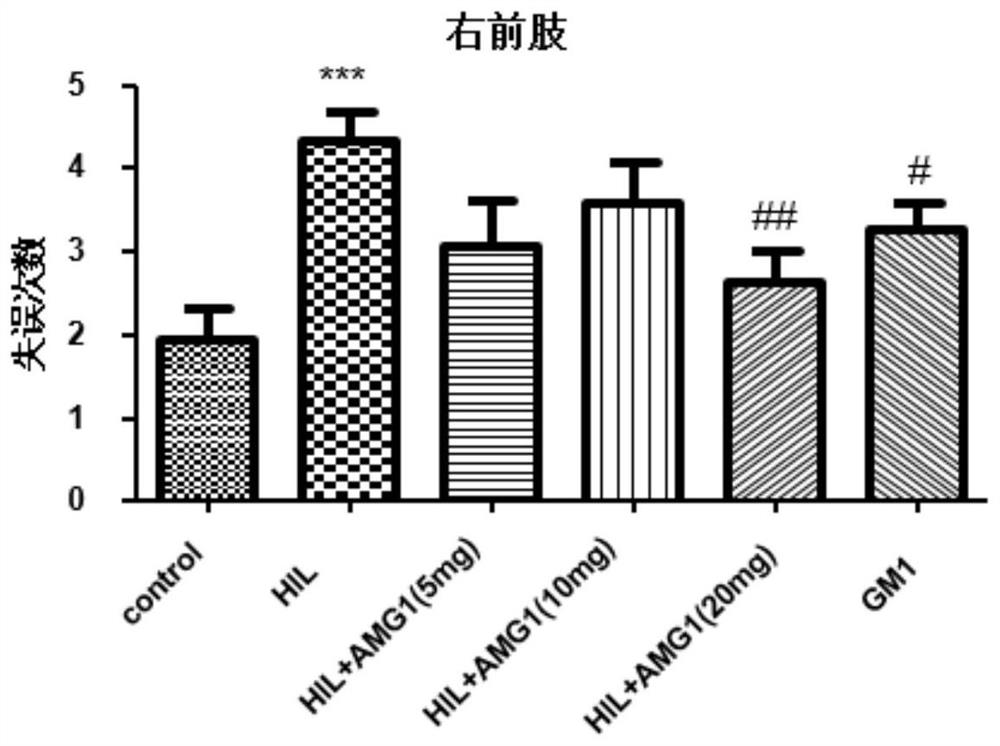
[0044] Effects of administration of α-mangostin derivatives on foot fault behavior test in rats with cerebral palsy.
[0045] Animal grouping and dosage are the same as in Example 1. The foot fault test is completed by a stainless steel mesh with a diameter of 0.4cm and a single grid area of 3cm*3cm. The mesh surface was raised about 1m from the ground. In order to reduce accidental errors, each rat was placed on the mesh for 2 minutes to adapt. The rats would step on the steel bars as much as possible, and the rats with poor balance would step on the air. Record the number of times the rats made mistakes with the right forelimb in 50 steps of grid crawling, and the number of times with the right hind legs in 50 steps in another 5 min.
[0046] The experimental results showed that in the right forelimb test, the number of right forelimb mistakes in the model group was significantly increased compared with the sham operation group (P<0.001), and the 20mg / kg administration gr...
Embodiment 3
[0048] To observe the effect of administration of α-mangostin derivatives on the submicroscopic structure of neurons in rats with cerebral palsy.
[0049] After the rats were anesthetized with chloral hydrate, the brain tissue was separated after perfusion with 4% paraformaldehyde (0.1M phosphate buffer, PH=7.4), and the sampling site was determined to minimize mechanical damage such as stretching, contusion and extrusion. The volume of the tissue generally does not exceed 1mm×1mm×1mm, and it is quickly put into the electron microscope fixative solution and fixed at 4°C for 2-4h. The cells were centrifuged until mung bean-sized cell agglomerates could be seen at the bottom of the tube, the culture medium was discarded and the electron microscope fixative was added to fix at 4°C for 2-4h. Rinse 3 times with 0.1M phosphate buffered solution PBS (PH7.4), 15 min each time. 1% osmic acid 0.1M phosphate buffer PBS (pH7.4) fixed at room temperature (20°C) for 2h. Rinse 3 times with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com