Nifedipine solid dispersion and preparation method thereof
A technology of dipine solid and nifedipine, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc., can solve the problems of low oral bioavailability, decreased solubility, slow drug release, etc. , to achieve the effect of improving bioavailability, bioavailability, and dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 nifedipine solid dispersion
[0043] 1, the preparation method of nifedipine solid dispersion, comprises the following steps:
[0044] S1. Weigh 300g of nifedipine and 700g of povidone PVP-VA64700g after passing through a 100-mesh sieve in the dark, and dry at 60°C for 6h;
[0045] S2. Mechanically mixing nifedipine and povidone PVP-VA64 in a closed container for 15 minutes to obtain a physical mixture;
[0046] S3. On the differential-speed asymmetric twin-screw extruder with the preset temperature of each section from the first to the sixth zone of the barrel at 150°C, 160°C, 165°C, 175°C, 175°C, and 165°C, single and double The head screw speed is 120 / 60r / min for extrusion, the extrudate is cooled and pulverized, and passed through an 80-mesh sieve to obtain the nifedipine solid dispersion.
[0047] 2. Results
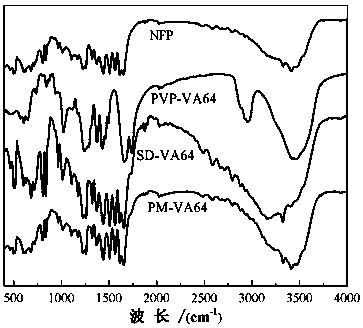
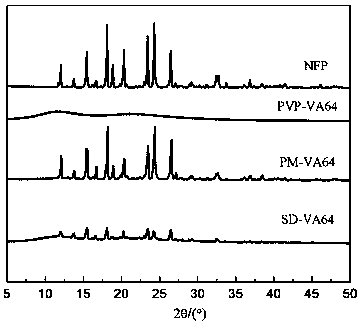
[0048] figure 1 and figure 2 Infrared spectrum and XRD spectrum of nifedipine (NEP), carrier povidone (PVP-VA64), physi...
Embodiment 2
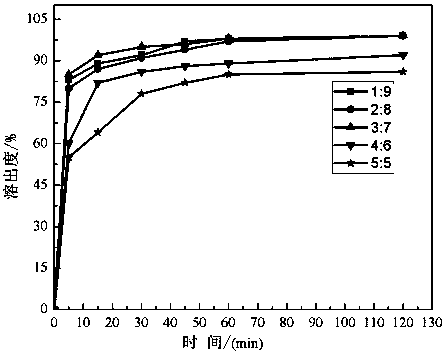
[0051] Embodiment 2 prepares nifedipine solid dispersion in different proportions
[0052] 1. Preparation method
[0053] Take by weighing respectively the nifedipine and PVP-VA64 of corresponding quality according to the ratio of table 1, then with reference to the preparation method of embodiment 1, nifedipine and PVP-VA64 are carried out mechanical mixing 15min in airtight container, obtain physical mixture; The pre-set temperature of each section from the first to the sixth zone of the barrel is 150°C, 160°C, 165°C, 175°C, 175°C, 165°C on the differential speed asymmetrical twin-screw extruder. Extrude at 120 / 60r / min, cool and pulverize the extrudate, and pass through an 80-mesh sieve to obtain nifedipine solid dispersions prepared with raw materials in different proportions.
[0054] The mass fraction of table 1 nifedipine and PVP-VA64
[0055] name
1
2
3
4
5
Nifedipine (g)
100
200
300
400
500
PVP-VA64(g)
900
8...
Embodiment 3
[0058] Embodiment 3 prepares nifedipine solid dispersion with common twin-screw extruder
[0059] 1, except that the differential speed asymmetric twin-screw extruder is replaced by a common twin-screw extruder, with reference to the method of embodiment 1, the preparation of nifedipine solid dispersion comprises the following steps:
[0060] S1. Weigh 300g of nifedipine and 700g of povidone PVP-VA64700g after passing through a 100-mesh sieve in the dark, and dry at 60°C for 6h;
[0061] S2. Mechanically mixing nifedipine and povidone PVP-VA64 in a closed container for 15 minutes to obtain a physical mixture;
[0062] S3. On an ordinary twin-screw extruder with preset temperatures of 150°C, 160°C, 165°C, 175°C, 175°C, and 165°C in each section of the barrel from zone 1 to zone 6, the screw speed is 120r / min extrusion, the extrudate was cooled and pulverized, and passed through an 80-mesh sieve to obtain a solid dispersion of nifedipine (SD-common).
[0063] 2. Results
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com