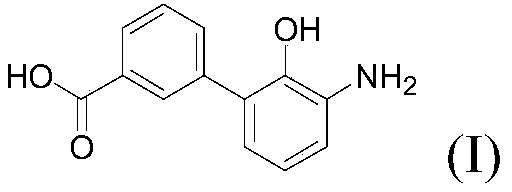
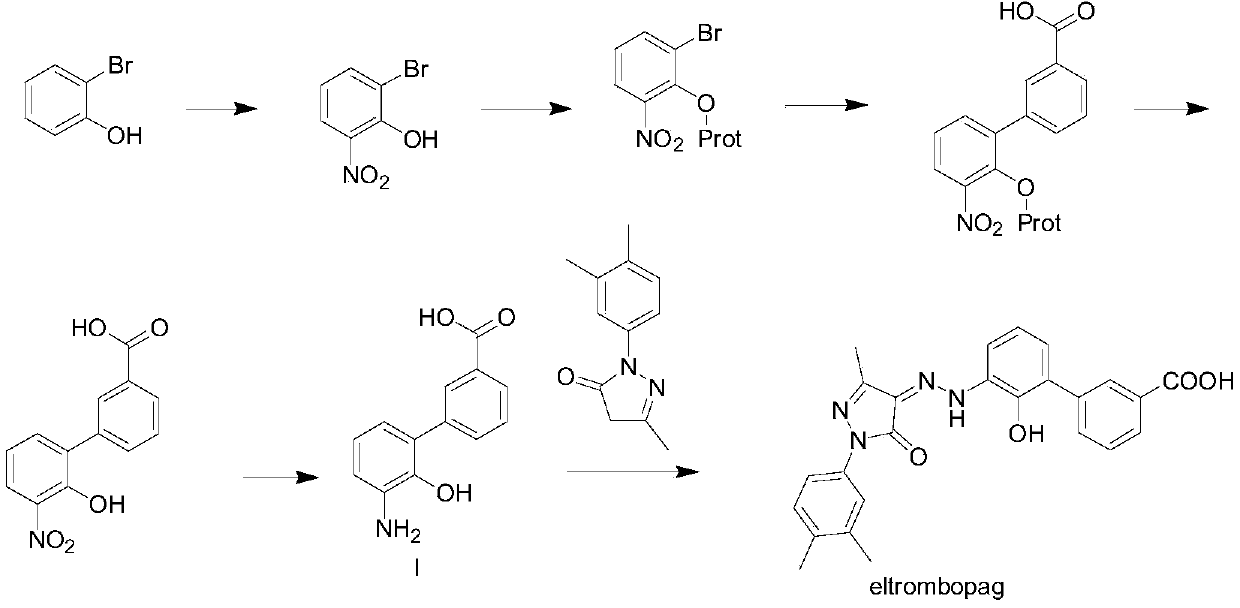
Preparation method of eltrombopag intermediate 2-hydroxy-3-(m-carboxyl phenyl)aniline
A carboxyphenyl and hydroxyl technology, which is applied in the field of preparation of Eltrombopag intermediate 2-hydroxy-3-aniline, can solve the problems of many side reactions, large equipment damage, and many waste acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] Provide a kind of preparation method of formula I compound 2-hydroxyl-3-(m-carboxyphenyl) aniline, described method comprises steps:
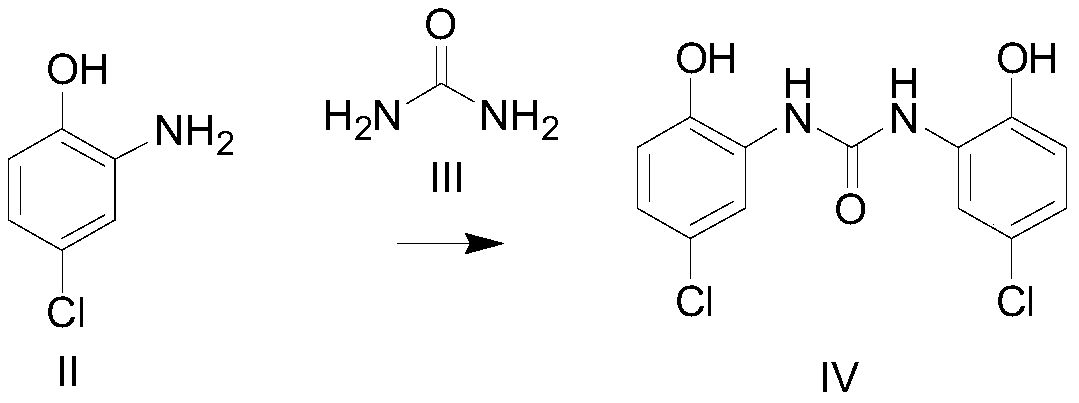
[0083] (1) Amino substitution reaction occurs between the compound of formula II and the compound of formula III, and the amino group is protected to obtain the compound of formula IV;
[0084]
[0085] (2) the compound of formula IV is subjected to bromination reaction to obtain the compound of formula V;
[0086]
[0087] (3) The benzylation reaction occurs between the compound of formula V and the compound of formula VI to obtain the compound of formula VII;
[0088]
[0089] (4) Suzuki coupling reaction occurs between the compound of formula VII and the compound of formula VIII to obtain the compound of formula IX;
[0090]
[0091] (5) hydrolyzing and deprotecting the compound of formula IX to obtain the compound of formula X; and
[0092]
[0093] (6) the compound of formula X removes benzyl and chlorine, obtains t...
Embodiment 1
[0113] Preparation of 1,3-bis-(5-chloro-2-hydroxyphenyl)-urea (IV)
[0114] Add 143.5g (1.0mol) 2-amino-4-chlorophenol (II), 1.4L chlorobenzene, 36g (0.60mol) urea (III) to a 3L reaction flask, heat up to 120-130°C with mechanical stirring, and react for 2h. Slowly cool down to 20-30°C, filter, wash the filter cake once with petroleum ether, and dry at 50°C to constant weight to obtain 148.7g (0.474mol) of compound 1,3-bis-(5-chloro-2-hydroxy Phenyl)-urea, yield 95%. C13H10Cl2N2O3, ESI-MS (m / z):
[0115] 313.03 [M+H], 312.01.
Embodiment 2
[0117] Preparation of 1,3-bis-(5-chloro-3-bromo-2-hydroxyphenyl)-urea (V)
[0118] Add 125.3g (0.40mol) 1,3-bis-(5-chloro-2-hydroxyphenyl)-urea (IV), 600ml acetic acid, 250ml tetrahydrofuran to a 1L reaction flask, stir mechanically to dissolve, and control the temperature for 20- 153.6g (0.96mol) of liquid bromine was added dropwise at 30°C, reacted for 3 hours, recovered tetrahydrofuran and acetic acid by distillation, added 500ml of water, stirred, filtered, and dried to obtain compound 1,3-bis-(5-chloro-3- Bromo-2-hydroxyphenyl)-urea 169.5g (0.36mol), yield 90%. C13H8Br2Cl2N2O3, ESI-MS (m / z): 469.76 [M+H], 467.83.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com