Lansoprazole sustained-release preparation
A slow-release preparation, the technology of lansoprazole, is applied in the directions of pill delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., to achieve the effects of improving drug compliance, avoiding influence, and reducing technical requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
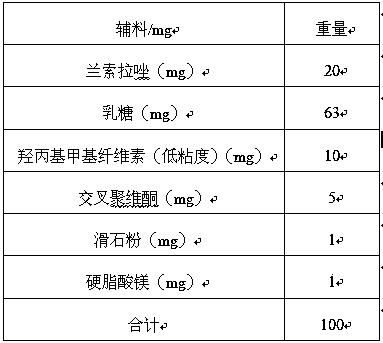
[0021] Tablet core prescription (mg):
[0022]
[0023] Coating layer prescription (mg):
[0024]
[0025] Preparation:
[0026] 1. Preparation of tablet core: take by weighing an appropriate amount of lansoprazole powder, add the auxiliary materials in the above table, pass through a 20 mesh sieve to make wet granules, dry at 50°C, dry the granules through a 15 mesh sieve for granulation, add 1% stearin Magnesium acid is mixed evenly, and tablet cores are obtained after tableting.
[0027] 2. Preparation of the coating layer: After mixing the auxiliary materials of the coating layer, pass through a 20-mesh sieve to make wet granules, dry at 50°C, pass the dry granules through a 20-mesh sieve for granulation, add 1% magnesium stearate, mix well, Made into coating material.
[0028] 3. Press coating: Weigh the prescribed amount of coating material and divide it into 2 parts, the ratio is 1:1. Fill a portion of the coating material into the die, place the tablet core i...
experiment example 1
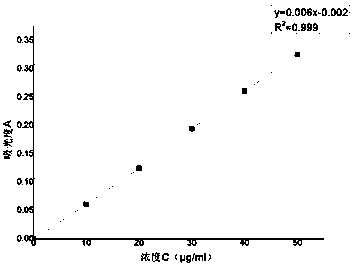
[0031] Experimental Example 1 Linearity and Range
[0032] Measure 0.4ml, 0.6ml, 0.8ml, 1.0ml, 1.2ml, 1.4ml of the reference substance stock solution respectively and place them in a 50ml measuring bottle, add pH6.8 buffer solution to constant volume and shake well. The absorbance values were measured at 284nm, the results are shown in Table 1-1
[0033] The absorbance of table 1-1 different concentration lansoprazole solution
[0034]
[0035] With the absorbance A as the ordinate and the concentration C as the abscissa, linear regression is carried out to obtain the standard curve, see
[0036] figure 1 .
experiment example 2
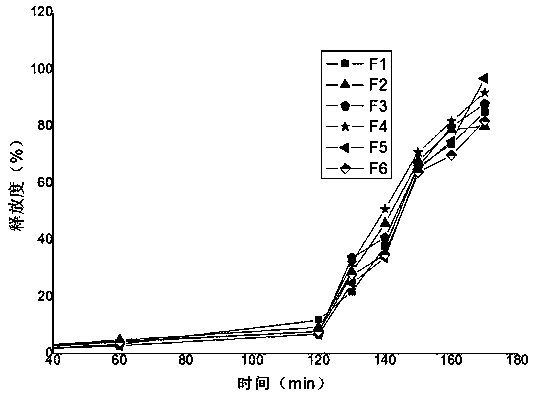
[0037] Physical evaluation of experimental example 2 tablet core:
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com