Preparation method and product of avian influenza vaccine
A technology of bird flu and bird flu virus, which is applied in the field of preparation of live bird flu vaccine, can solve the problems of wasting the immune potential of the body, and achieve the effects of improving equipment utilization, good safety, and saving equipment space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
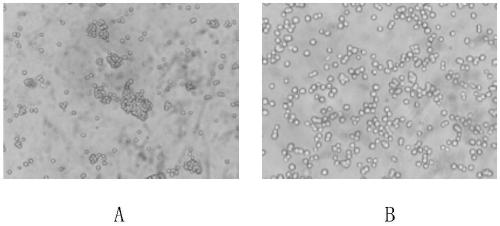
[0030] Example 1 Acclimatization of DF-1 cells adapted to the serum-free medium and growing in a suspension state The DF-1 cells were acclimatized by gradually lowering the serum to adapt to the culture at low concentration of serum. When DF-1 cells growing stably in MEM medium containing 10% newborn bovine serum grow to the mid-logarithmic growth phase, replace them with MEM medium containing 5% newborn bovine serum until the cells grow to 80%-90% At confluence, trypsinize the cells to a cell density of 2.0 x 10 5 The cells / ml were subcultured in MEM medium containing 5% newborn bovine serum. After several generations, the survival rate of DF-1 cells in the MEM medium containing 5% newborn bovine serum was maintained at more than 90%, and maintained a relatively fast growth rate, which was used to further reduce serum acclimation culture. In the same way, DF-1 cells were gradually adapted to the culture condition of MEM containing 1% newborn bovine serum. The DF-1 cells ada...
Embodiment 2
[0032] The preparation of embodiment 2 bird flu vaccines
[0033] 1. The DF-1-XF cells obtained in Example 1 were domesticated and cultured according to the ratio of 0.75×10 6 cells / ml is the initial inoculation density of the cells, inoculated into shaker flasks, and carried out shaking culture in a shaker. After 48 hours of culture, let the cells settle. Discard the supernatant after the cells have completely settled, and add the same volume of virus growth solution; press The avian influenza virus was inoculated with a dose of MOI of 0.01, and TPCK-pancreatin at a final concentration of 4 μg / ml was added at the same time, and the virus liquid was harvested after culturing for 72 hours. Other suspension culture conditions: pH control is 7.2, dissolved oxygen (DO) is 50%, temperature is 36°C, stirring speed is 100r / min;
[0034] 2. Inactivation
[0035] Add the virus liquid that has passed the sterility test into the formaldehyde solution, so that the final concentration of...
Embodiment 3
[0049] The preparation of embodiment 3 bird flu vaccines
[0050] 1. The DF-1-XF cells obtained in Example 1 were domesticated and cultured according to the ratio of 0.75×10 6 cells / ml is the initial inoculation density of the cells, inoculate them into shaker flasks, and carry out shaking culture in a shaker. After 36 hours of culture, let the cells settle. Discard the supernatant after the cells have completely settled, and add the same volume of virus growth solution; press Avian influenza virus was inoculated at an MOI of 0.0001, and TPCK-pancreatin at a final concentration of 8 μg / ml was added at the same time, and the virus liquid was harvested after culturing for 72 hours. Other suspension culture conditions: pH control is 7.2, dissolved oxygen (DO) is 50%, temperature is 36°C, stirring speed is 100r / min;
[0051] 2. Inactivation
[0052] Add the virus liquid that has passed the sterility test into the formaldehyde solution, so that the final concentration of the form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com