Preparation method and application of carboxypeptidase A with function of degrading ochratoxin A
A technology of ochratoxin and carboxypeptidase, which is applied in the field of preparation of mature peptide of carboxypeptidase A, can solve the problems of complex preparation methods, reduced catalytic activity, and harsh catalytic conditions, and achieve high-efficiency and stable degradation, good application potential, and The effect of good environmental adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Codon Optimization of Carboxypeptidase A Mature Peptide Encoding Gene
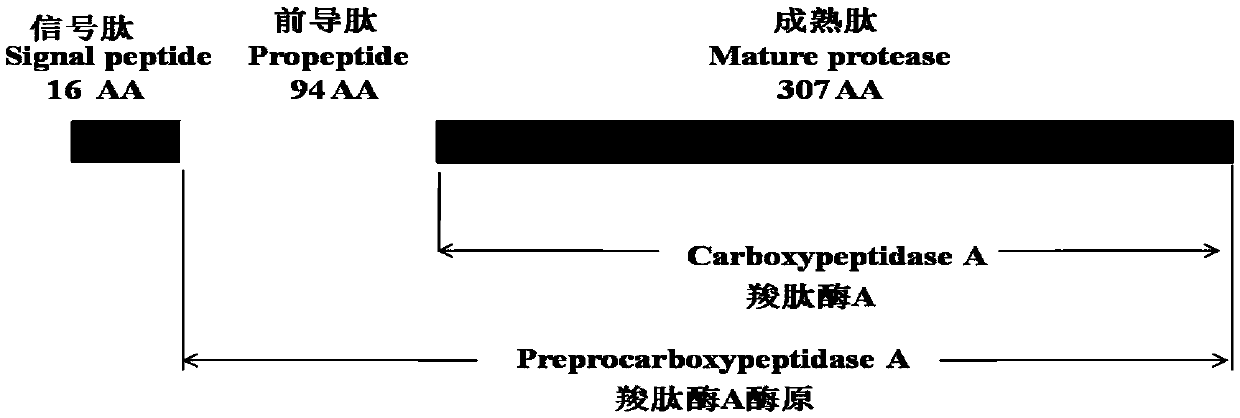
[0049] First, the secondary and tertiary structures of bovine pancreatic carboxypeptidase A were analyzed using bioinformatics software. After a large number of screenings of different protein truncated expression modes, the carboxypeptidase containing 307 amino acids was determined to remove the C-terminal signal peptide and leader peptide. The truncated expression of peptidase A mature peptide (M-CPA), the sequence structure of the pro-CPA enzyme is shown in figure 1 shown. The amino acid sequence of the mature peptide of bovine pancreatic carboxypeptidase A (M-CPA) is shown in SEQ ID NO.1, and the nucleotide sequence of the coding gene is shown in SEQ ID NO.3. According to the codon preference of Pichia pastoris, combined with codon optimization software and manual screening, the coding gene of the codon-optimized CPA mature peptide shown in SEQ ID NO.2 was obtained, and the coding gen...
Embodiment 2
[0050] Embodiment 2 expresses the carrier of carboxypeptidase A mature peptide and the construction of recombinant yeast
[0051] The gene encoding the mature CPA peptide obtained in Example 1 was connected between the EcoR I and Not I restriction sites of the pPIC9K plasmid to construct the Pichia pastoris expression vector pPIC9K / M-CPA. The expression vector was transformed into Pichia pastoris GS115 (Pichia pastoris GS115), and the positive recombinant strain was obtained through screening, and the recombinant Pichia strain in which the pPIC9K / M-CPA vector was stably integrated into the chromosome was obtained through passage.
Embodiment 3
[0052] The cultivation of embodiment 3 recombinant Pichia pastoris and the preparation of carboxypeptidase A
[0053] This example provides a method for culturing and inducing expression of recombinant Pichia pastoris for preparing a mature peptide of carboxypeptidase A, specifically as follows:
[0054] (1) Activation culture on a solid plate: the recombinant Pichia pastoris strain constructed in Example 2 was inoculated on a fresh YPD plate, and cultured upside down in a yeast incubator at 30° C. for 2 days.
[0055] (2) Seed culture: Pick single colonies of the recombinant strains, inoculate them in 25mL BMGY medium (containing 4% glycerol), and cultivate them in a 250mL Erlenmeyer flask until the cell concentration reaches OD 600 =2~3. The culture conditions are: 28°C, 225r / min shaker culture.
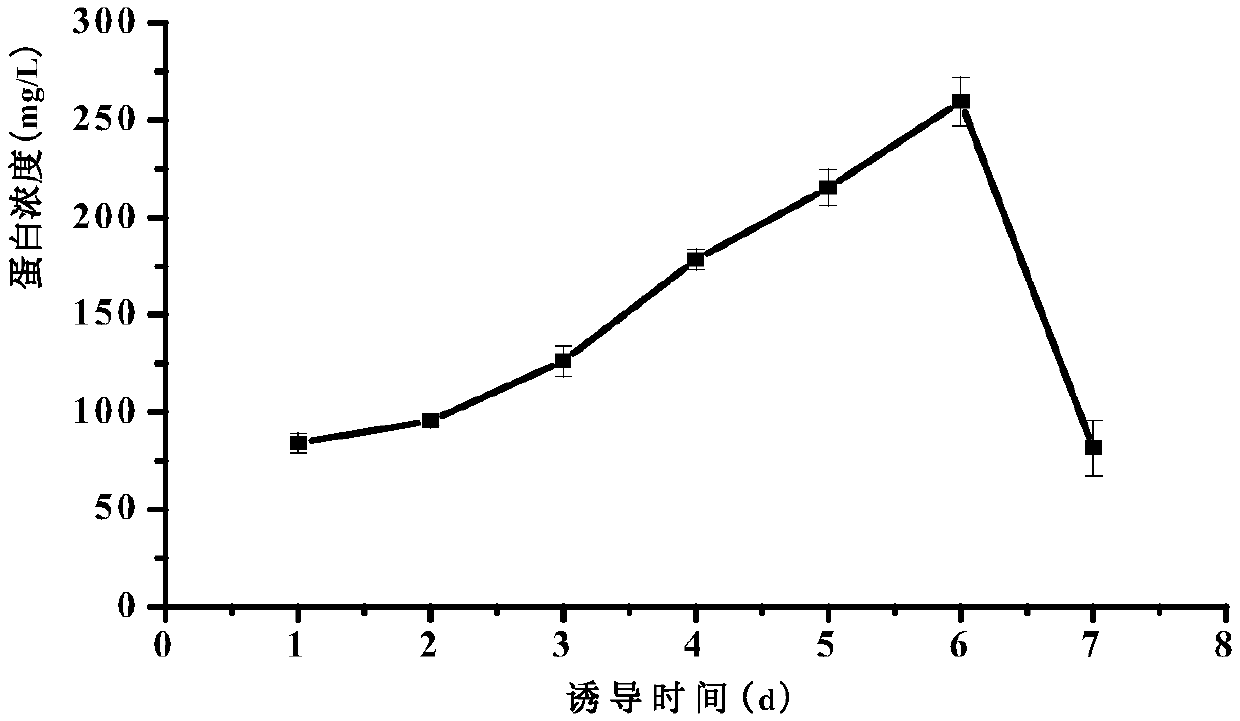
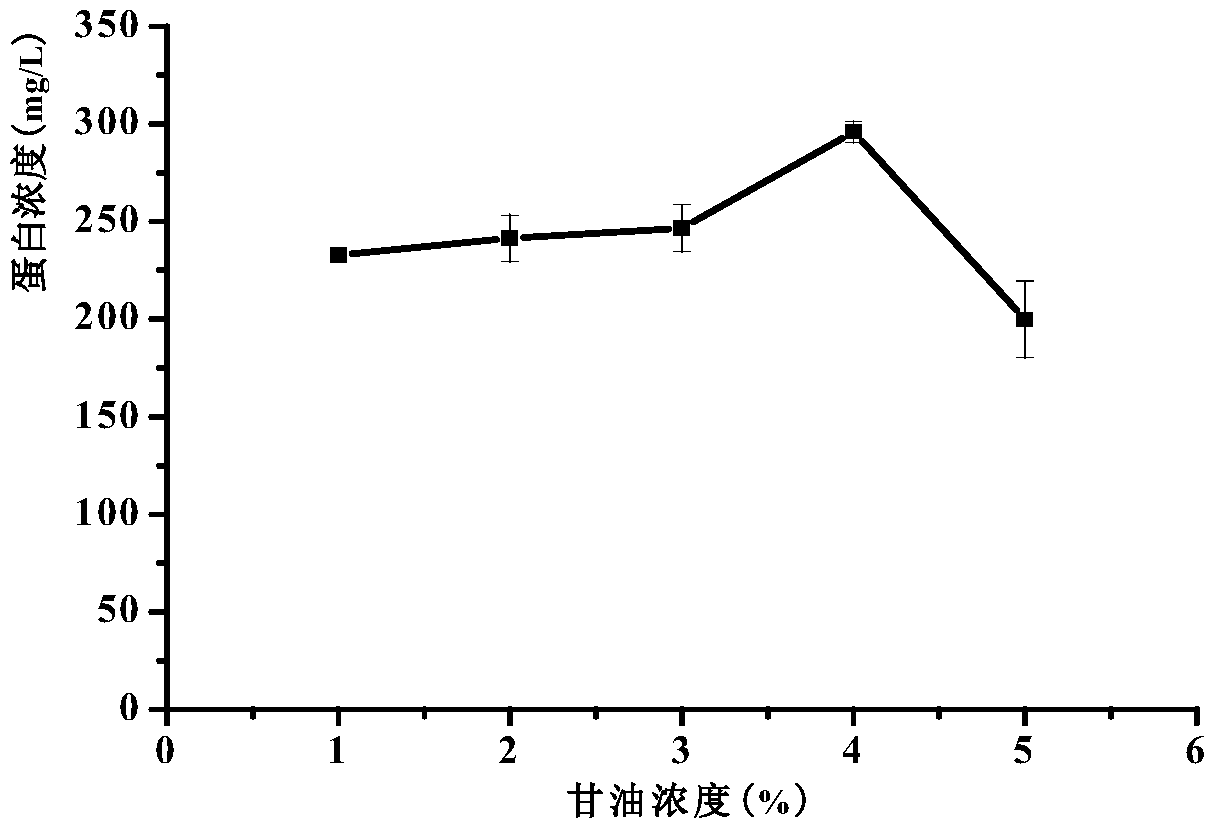
[0056] (3) Induced expression: collect the above culture, centrifuge at room temperature at 4000rpm for 5min, pour off the supernatant, and resuspend the obtained bacterial cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com