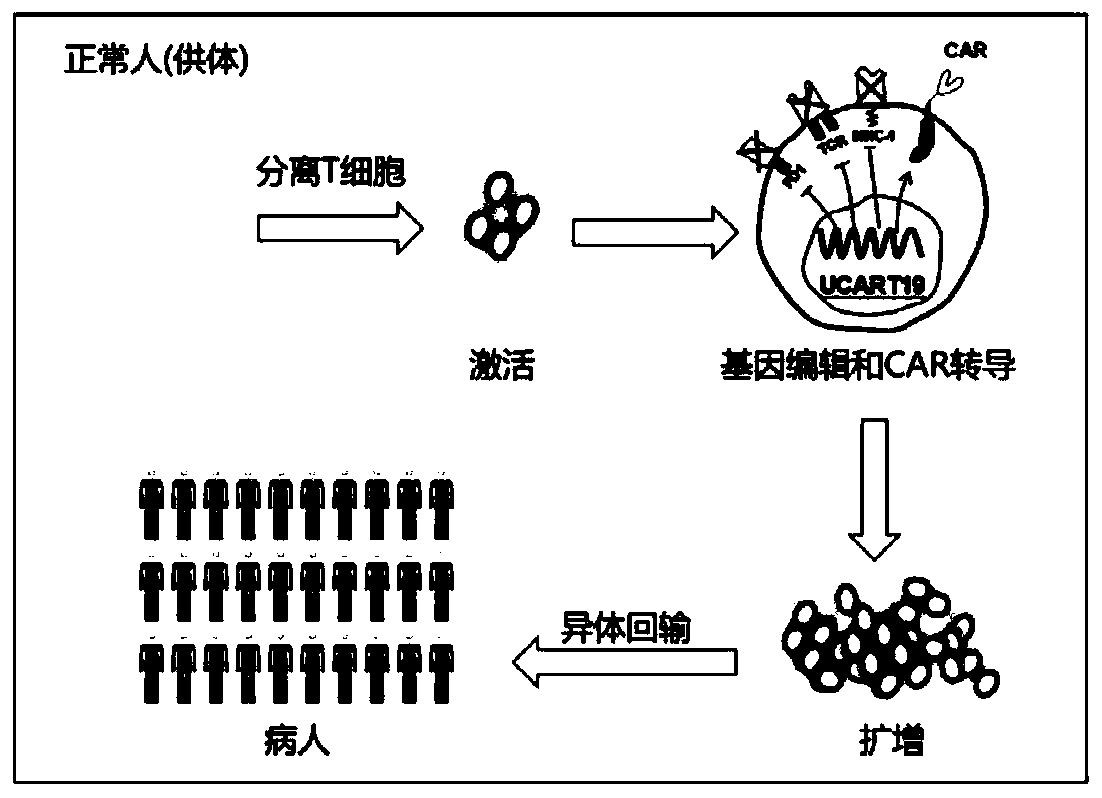
A kind of function-enhanced universal car-t cell and its preparation method and application
A general-purpose, cell-based technology, applied in the fields of medicine, immunotherapy, biological cytology and molecular biology, can solve the problems of high cost and high price, and achieve the effect of improving function, strong lethality, and restraining inhibitory signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Construction of Single Gene Knockout Lentiviral Vector
[0063] a. Design Oligo DNA primers: for the first exon sequence of T cell receptor TRAC (α chain) and TRBC (β chain) gene, PD1 receptor gene signal peptide region and Ig-like V-Type region, B2M Oligo DNA primers were designed for the second exon of the gene, and the primer sequences are shown in Table 2:
[0064] Table 1 sgRNA sequence list for single gene knockout
[0065]
[0066] Table 2 Oligo DNA primers
[0067]
[0068]
[0069] b. Anneal and phosphorylate Oligo1 and Oligo2 respectively to form double-stranded DNA. The annealing and phosphorylation system is as follows:
[0070]
[0071] Step down program setting: 37°C for 30min, 95°C for 5min, 90°C for 1min, 85°C for 1min, 80°C for 1min, 75°C for 1min, 70°C for 1min, 65°C for 1min, 60°C for 1min, 55°C for 1min , 50°C 1min, 45°C 1min, 40°C 1min, 35°C 1min, 30°C 1min, 25°C 1min.
[0072] c. The reaction system was diluted 200 times a...
Embodiment 2
[0077] Example 2 Construction of Multiple Gene Knockout Lentiviral Vectors
[0078] The PTG (Polycistronic tRNA-gRNA) structure is a sequence structure in which multiple gRNAs are spaced in series, and the PTG structure can be inserted downstream of the U6 promoter in the LentiCRISPRv2 vector. The PTG sequence of two sgRNAs in series and three sgRNAs in series is inserted into the gene knockout lentiviral vector LentiCRISPRv2, so as to obtain a gene knockout vector that expresses multiple sgRNAs at the same time, thereby achieving the purpose of knocking out multiple genes at the same time. figure 2 Map and elements for the Lenti-PTG-4 lentiviral vector.
[0079] Table 4 The sgRNA sequence information table contained in the PTG of multiple gene knockout
[0080]
[0081] Table 5 PTG sequence list of multiple gene knockout
[0082]
[0083]
[0084] Table 6 Multiple gene knockout lentiviral vectors
[0085]
Embodiment 3
[0086] Example 3 Construction of anti-CD19CAR lentiviral vector
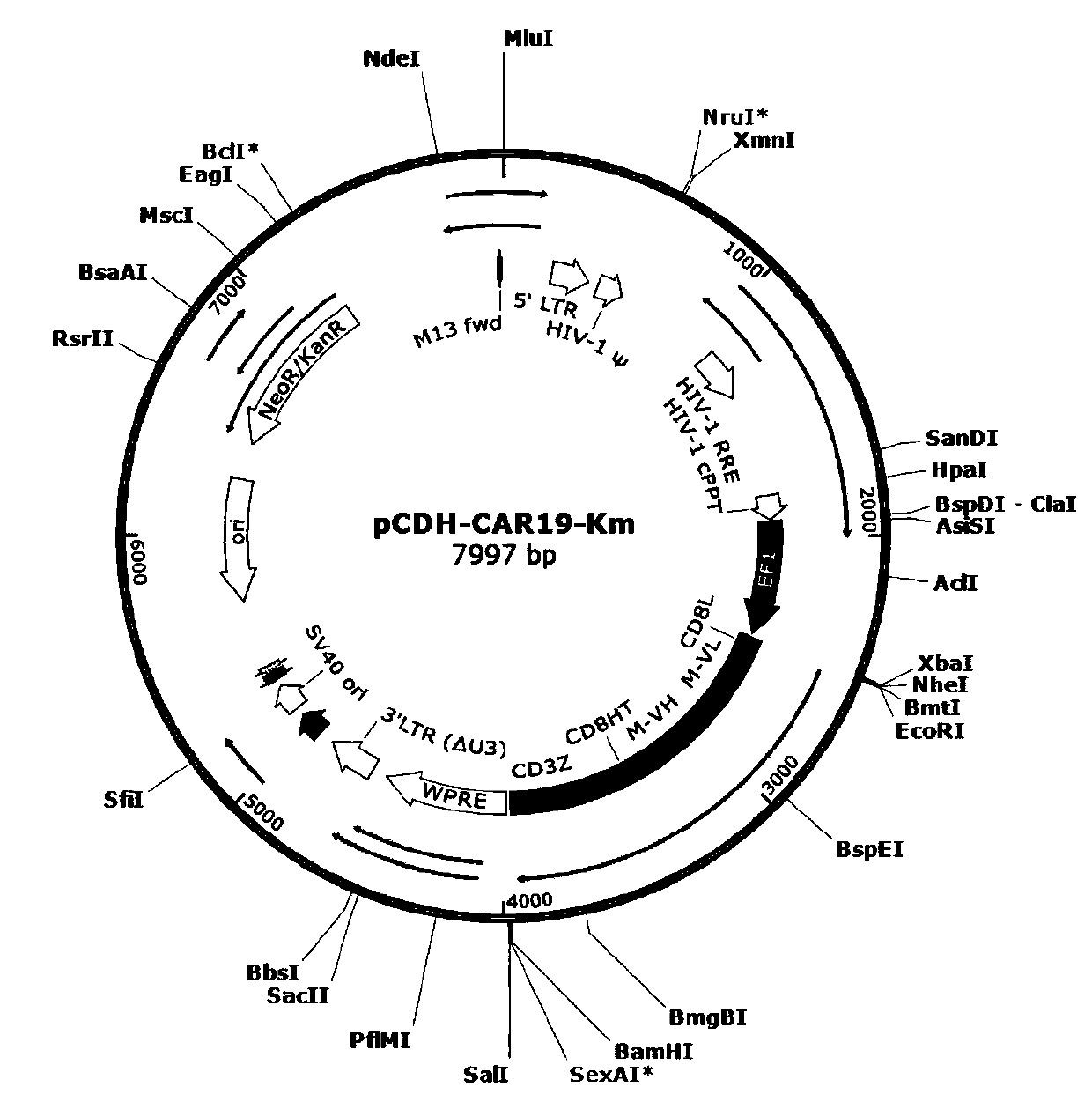
[0087] The anti-CD19 chimeric antigen receptor (CAR) structure includes a CD19 antigen-binding region (derived from the single-chain variable region scFv of the murine monoclonal CD19 antibody FMC63), a CD8α extracellular hinge region, a CD8α transmembrane region, and a 4-1BB intracellular co-stimulatory domain and a CD3ζ activation signal domain, its amino acid sequence is shown in SEQ ID NO: 16, and its nucleic acid sequence is shown in SEQ ID NO: 17. The full length of the sequence is 1458bp, with a total of 486 amino acids. The amino acid sequence of the CAR fusion protein was optimized by the codons of the human species to obtain a nucleic acid sequence, including the N-terminal kozak sequence (GCCGCCACC) and the C-terminal TAA stop codon. The CAR fusion gene sequence is located between the restriction endonucleases EcoRI and BamHI to obtain the full plasmid nucleic acid sequence (7905bp) of the lentiviral...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com