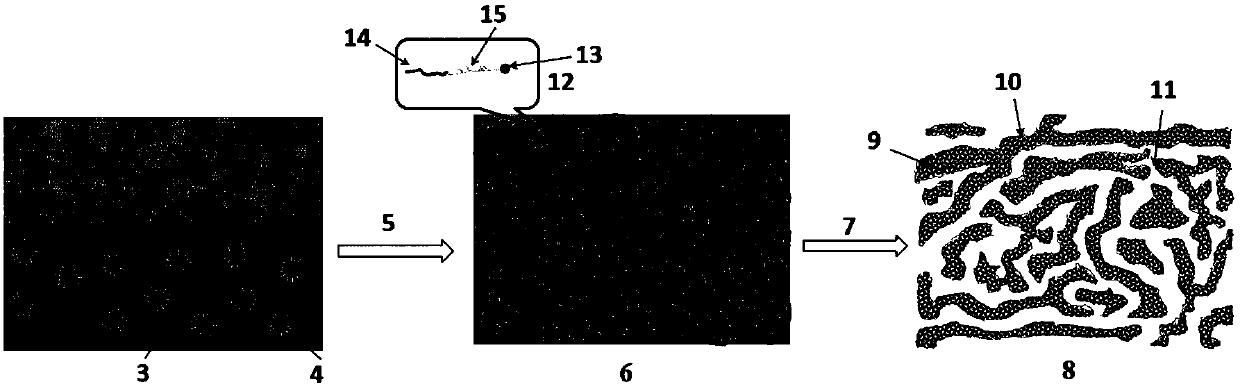
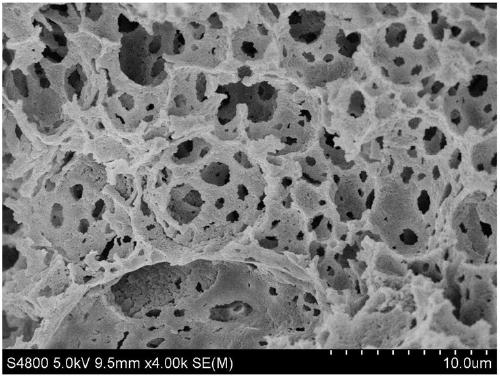
Method for preparing double-channel hydrophilic bicontinuous polymer monolithic column
A polymer and hydrophilic technology, applied in the field of dual-channel hydrophilic bicontinuous polymer monolithic column and its preparation, can solve the problems of poor mechanical strength, low separation efficiency, poor biocompatibility, etc., to ensure uniformity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1) Synthesis of poly 3-O-methacryloyl-diacetone-D-glucose (PMDAGlu) by ATRP reaction
[0069] Add a stirring bar to the Schlenk bottle at room temperature, then add cuprous bromide (5.9mg), N,N,N,N',N'-pentamethyldiethylenetriamine (PMDETA) (7.1mg ), MDAGlu (0.98g) and 3ml chlorobenzene, through three cycles of liquid nitrogen freezing-pumping-gassing-thawing to remove oxygen. Finally, the initiator 2-bromoisobutyrate (30.4 mg) was added, reacted at 50° C. for 4 h, dissolved the reaction product with tetrahydrofuran, diluted the reaction product, and removed the catalyst through an aluminum oxide column. The obtained colorless solution was precipitated twice with n-hexane, suction filtered at room temperature, and dried in vacuo to obtain 0.6 g of PMDAGlu, whose molecular weight Mn=4700 was detected by gel permeation chromatography (GPC).
[0070] 2) Synthesis of poly 3-O-methacryl-diacetone-D-glucose-polystyrene block copolymer (PMDAGlu-PS) by ATRP reaction
[0071] ...
Embodiment 2
[0075] 1) Synthesis of 3-O-acryloyl-diacetone-D-glucose (PDAGlu) by RAFT polymerization
[0076] Add a stirring bar to a 5mL chicken heart bottle at room temperature, then add sugar-containing monomer DAGlu (0.7g), chain transfer agent CPADB (30mg), initiator AIBN (2mg) and solvent dioxane (1mL) in sequence, dissolve and mix After uniformity, use argon purging to remove oxygen in the system. Then react at 75°C for 15 hours, dilute the reaction solution with tetrahydrofuran, precipitate with n-hexane two to three times, and use centrifugation to obtain a solid product, dry it in vacuum at room temperature to obtain 0.37g PDAGlu, and determine the molecular weight M by GPC. n =3450.
[0077] 2) Synthesis of PDAGlu-PS by RAFT polymerization
[0078] Add and stir once in the 10mL heart bottle at room temperature, then add one block polymer PDAGlu (0.3g), initiator AIBN (2.8mg), styrene (3.2g) and solvent dioxane (3mL) successively, use Argon was purged to remove oxygen, and fin...
Embodiment 3
[0084] 1) Synthesis of 3-O-acryloyl-diacetone-D-galactose (PADAGlu) by ATRP reaction
[0085] Add a stirring bar to the Schlenk bottle at room temperature, then add cuprous bromide (5.48mg), N,N,N,N',N'-pentamethyldiethylenetriamine (PMDETA) (7.65mg ), 3-O-methacryloyl-diacetone-D-galactose (MDAGal) (1.30g) and 3.5ml toluene, through three cycles of liquid nitrogen freezing-pumping-gassing-thawing to remove oxygen. Finally, the initiator 1-bromoethylbenzene (18.3 mg) was added, reacted at 60° C. for 4 h, dissolved the reaction product with chloroform, diluted the reaction product, and removed the catalyst through an aluminum oxide column. The resulting colorless solution was precipitated twice with methanol, suction filtered at room temperature, and dried in vacuo to obtain 0.69 g of PMDAGal, whose molecular weight Mn=6800 was detected by gel permeation chromatography (GPC).
[0086] 2) Use ATRP reaction to synthesize 3-O-acryloyl-diacetone-D-glucose-polystyrene block copolym...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com