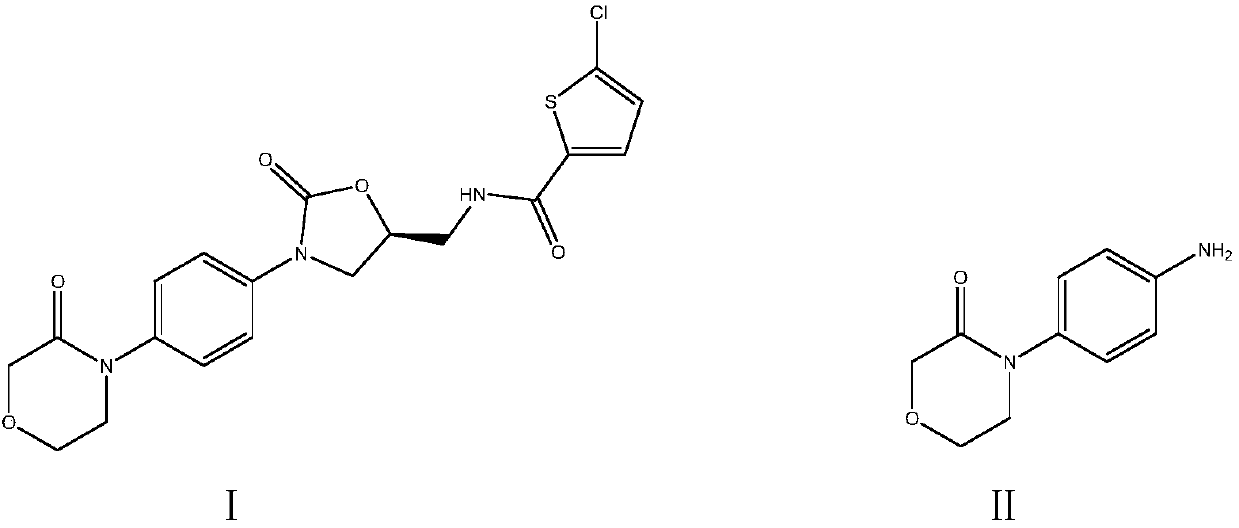
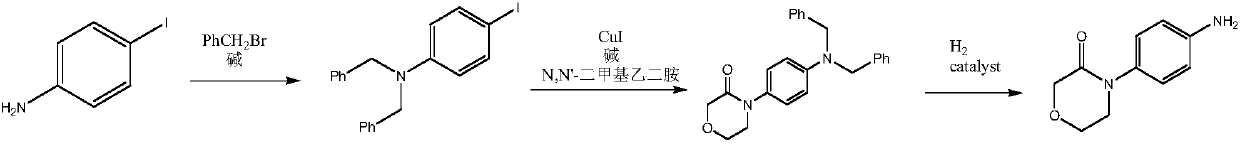
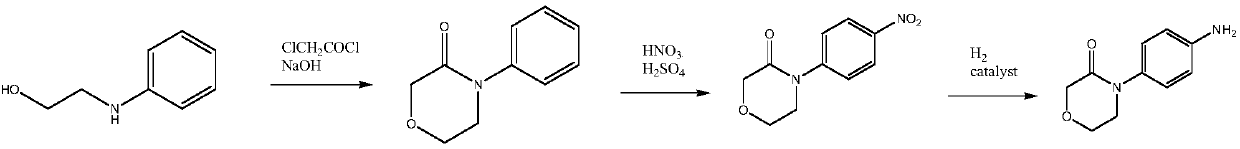
Synthetic method of high-selectivity 4-(4-aminophenyl) morpholine-3-one
An aminophenyl, high-selectivity technology, applied in the synthesis of 4-morpholin-3-one and the synthesis of morpholin-3-one derivatives, can solve the problems of being unfavorable for the preparation of high purity and high activity, and achieve The effect of avoiding competing side reactions, high reaction selectivity, and few separation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: the preparation of 4-(4-aminophenyl) morpholin-3-ketone (II)
[0065] In a 500 ml four-necked flask connected with a stirrer, a thermometer, and a condenser tube, add 70.5 grams (0.5 moles) of p-nitrofluorobenzene, 266.5 grams (2.0 moles) of methyl 2-aminoethoxyacetate, and 75.0 grams of carbonic acid Potassium, heating, stirring at 100-105°C for 4 hours (condensation reaction), cooling to 20-25°C, filtering, and washing the filter cake twice with dichloromethane, 50 grams each time. Combine the filtrates, the filtrate is first atmospheric distillation to reclaim methylene chloride, then vacuum distillation to reclaim 2-aminoethoxyacetic acid methyl ester, and the residue is 128.2 grams of 2-(4-nitrophenyl) aminoethoxyacetic acid methyl ester (V1), the yield is 100%, and the GC purity is 99.8%, which is directly transferred to 500 milliliters of stainless steel autoclave, and the former flask is washed with 300 gram of methanol, and the washing liquid is t...
Embodiment 2
[0069] Embodiment 2: Preparation of 4-(4-aminophenyl) morpholin-3-ketone (II)
[0070] In a 250 ml four-necked flask connected with a stirrer, a thermometer, and a condenser tube, add 14.1 grams (0.1 moles) of p-nitrofluorobenzene, 73.5 grams (0.5 moles) of ethyl 2-aminoethoxyacetate, and 15.0 grams of carbonic acid Potassium, heating, stirring at 100-105°C for 3 hours, cooling to 20-25°C, filtering, and washing the filter cake twice with dichloromethane, 50 grams each time. Combine the filtrates, the filtrate is first atmospheric distillation to reclaim dichloromethane, then vacuum distillation to reclaim 2-aminoethoxy ethyl acetate, and the residue is 27.1 grams of 2-(4-nitrophenyl) aminoethoxy ethyl acetate (V2), the yield is 100%, and the GC purity is 99.7%, which is directly transferred to 500 milliliters of stainless steel autoclave, and the former flask is washed with 100 grams of ethanol, and the washing liquid is transferred to the autoclave together, and 5.0 grams of...
Embodiment 3
[0071] Embodiment 3: Preparation of 4-(4-aminophenyl) morpholin-3-ketone (II)
[0072] In the 250 milliliter four-necked flask that is connected with stirrer, thermometer, condenser tube, add 15.8 grams (0.1 moles) p-nitrochlorobenzene, 80.5 grams (0.5 moles) isopropyl 2-aminoethoxyacetate, 15.0 grams Potassium carbonate, heated, stirred at 100-105°C for 3 hours, cooled to 20-25°C, filtered, and the filter cake was washed twice with dichloromethane, 50 grams each time. Combine the filtrates, the filtrate is first normal pressure distillation to reclaim methylene chloride, and then vacuum distillation to reclaim 2-aminoethoxyacetic acid isopropyl ester, and the residue is 28.2 grams of 2-(4-nitrophenyl) aminoethoxyacetic acid isopropyl ester. Propyl ester (Ⅴ 3), yield is 100%, GC purity is 99.8%, is directly transferred to in the stainless steel autoclave of 500 milliliters, and washes former flask with 100 gram isopropanols, and washing liquid is transferred in the autoclave t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com