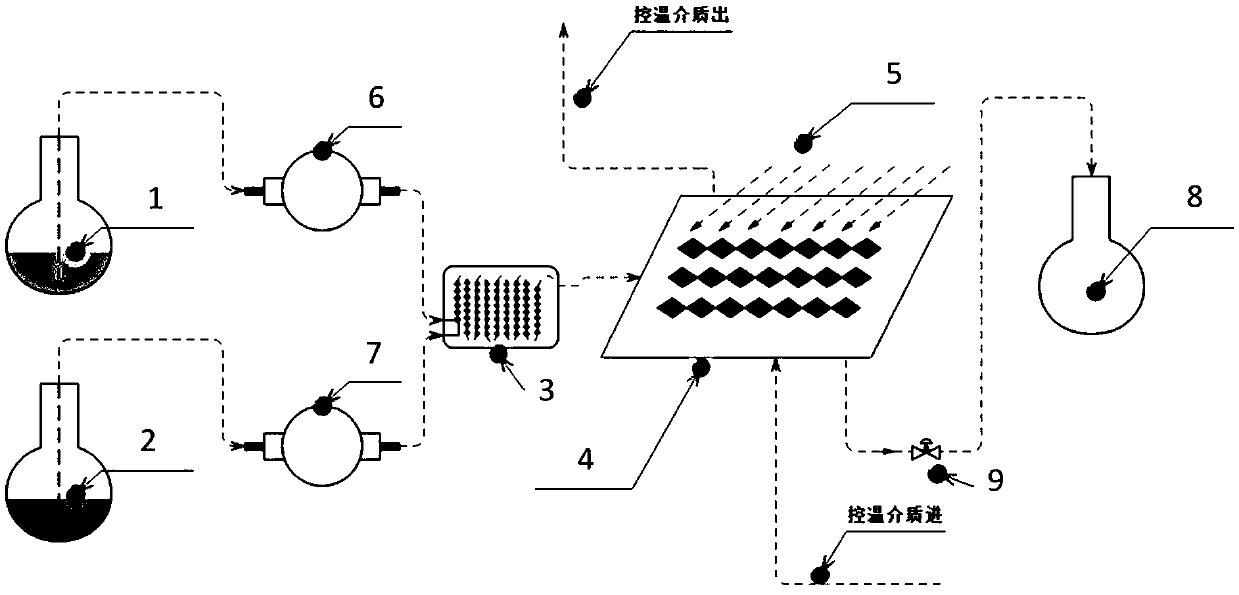
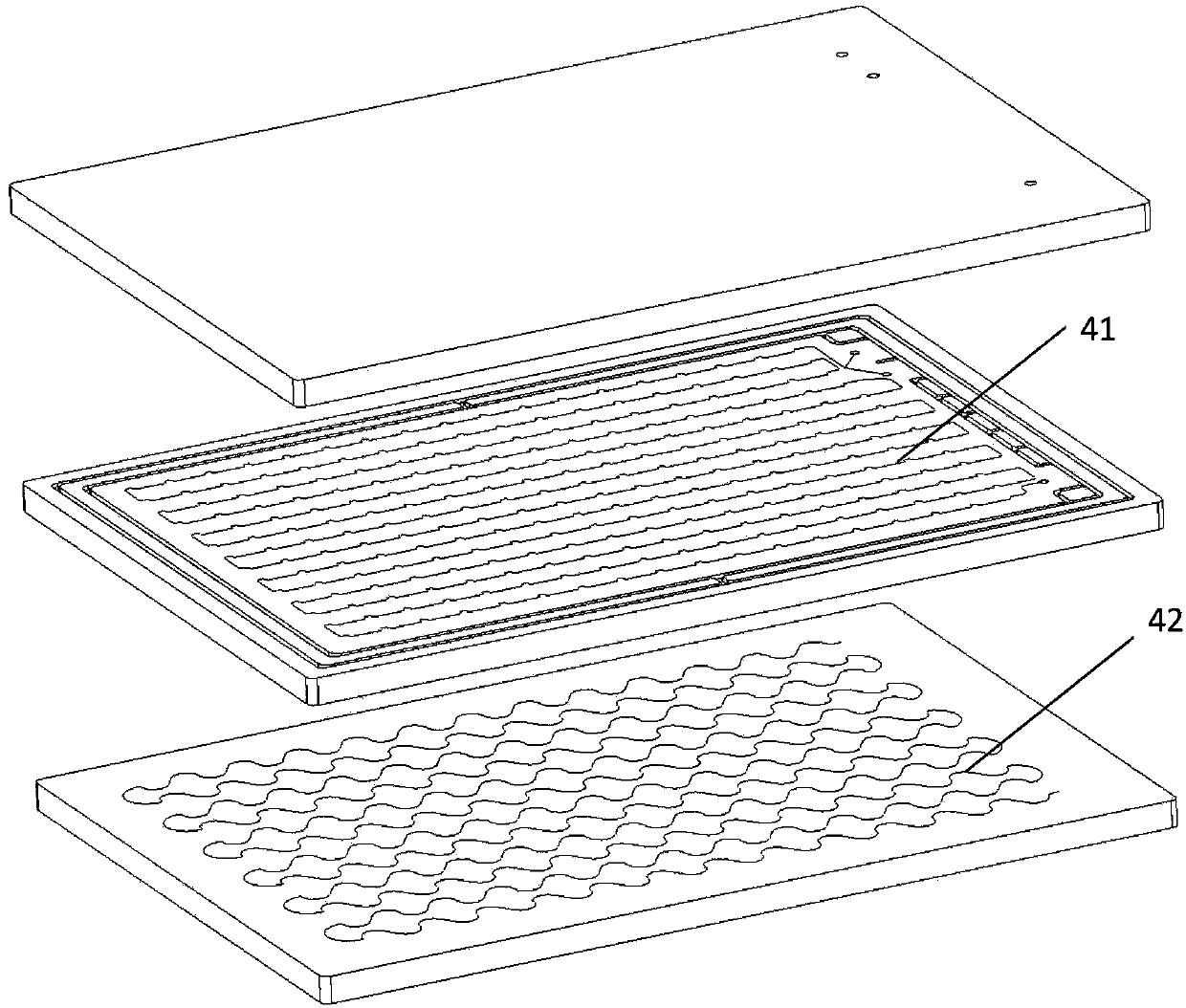
Reacting device used for benzyl brominating of biphenyl derivative and brominating method
A reaction device and derivative technology, applied in the field of biphenyl derivative bromination, can solve the problems of increased difficulty in post-processing, safety risks, low light absorption efficiency, etc., to avoid the use of free radical initiators and facilitate production Scale, the effect of adjusting the production scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
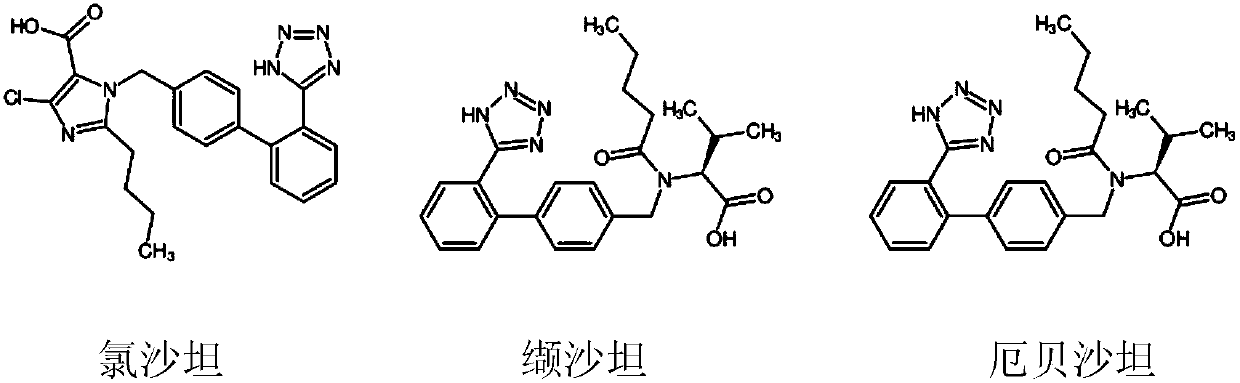
[0118] Dissolve 60g (0.311mol) of 2-cyano-4'methyl biphenyl in 360ml of dichloromethane, stir to dissolve, then weigh 27.84g (0.174mol) of elemental bromine and dissolve it in 2-cyano-4'methyl Stir evenly in the biphenyl solution, as a reaction solution, and store in one of the flasks (the first storage tank); hydrogen peroxide is stored in another flask (the second storage tank); set the first feed pump The flow rate is 22mL / min; the flow rate of the second feed pump is 5mL / min; turn on the LED light and feed pump, control the residence time of the material for 5mins, use water as the temperature control medium, and control the bromination temperature to 25°C-40°C; Collect the reacted materials discharged from the photocatalytic temperature-controlled pipeline reactor with a 500mL four-necked bottle, and quench the remaining elemental bromine and hydrogen peroxide with aqueous sodium sulfite. The layers were separated, the organic layer was spin-dried, and 75.6 g of the produ...
Embodiment 2
[0120] Dissolve 60g (0.125mol) of N-(triphenylmethyl)-5-(4'-methylbiphenyl-2-yl)tetrazolium in 360ml of dichloromethane, stir to dissolve, then weigh the elemental bromine 11g (0.069mol) was dissolved in N-(triphenylmethyl)-5-(4'-methylbiphenyl-2-yl)tetrazolium solution and stirred evenly, as a reaction solution, and stored in one of the flasks (the first storage tank); the hydrogen peroxide is stored in another flask (the second storage tank); the flow rate of the first feed pump is set to 22mL / min; the flow rate of the second feed pump is 5mL / min ;Turn on the LED light and feed pump, control the residence time of the material to 7mins, use water as the temperature control medium, and control the bromination temperature to 25°C-40°C; use a 500mL four-necked bottle to collect After the reaction, the remaining elemental bromine and hydrogen peroxide were quenched with an aqueous solution of sodium sulfite. The layers were separated, the organic layer was spin-dried, and 64.1 g...
Embodiment 3
[0122] Dissolve 60g (0.224mol) of tert-butyl 4'-methyl-[1,1'-biphenyl]-2-carboxylate in 300ml of dichloromethane, stir to dissolve, then weigh 19.7g (0.123mol) of elemental bromine Dissolve in 4'-methyl-[1,1'-biphenyl]-2-formic acid tert-butyl ester solution and stir evenly as a reaction solution, and store in one of the flasks (the first storage tank); hydrogen peroxide Store in another flask (the second storage tank); set the flow rate of the first feed pump to 22mL / min; the flow rate of the second feed pump to 5mL / min; turn on the LED light and feed pump, and the material stays The time is 9mins, water is used as the temperature control medium, and the bromination temperature is controlled at 25°C-40°C; the reacted materials discharged from the photocatalytic temperature-controlled pipeline reactor are collected in a 500mL four-necked bottle, and the remaining Elemental bromine and hydrogen peroxide. Separate the layers, spin dry the organic layer, and recrystallize from a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com