Whole-cell one-step method for synthesizing L-carnosine
A whole-cell, carnosine technology, applied in the field of carnosine synthesis, can solve problems such as unfavorable large-scale application, low L-carnosine yield, low L-carnosine yield, etc., to solve the problem of enzyme separation and purification, high cost and molar conversion rate High, simple reaction system effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention will be further described in detail below in conjunction with the accompanying drawings and specific embodiments.
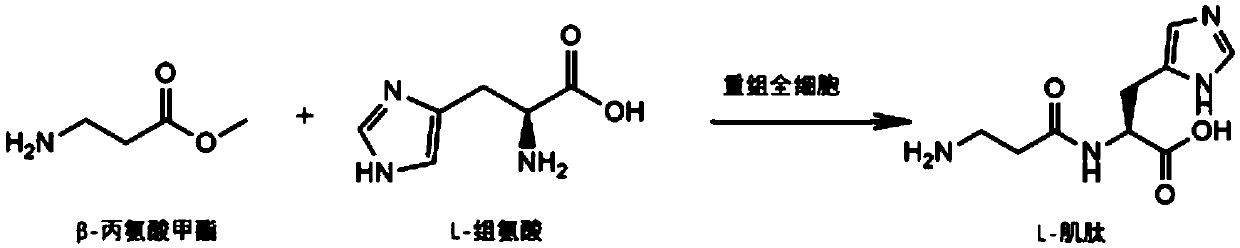
[0028] The L-carnosine biosynthetic pathway of the present invention is briefly described as such figure 1 As shown, it specifically includes the following steps:
[0029] 1. Construction of amino acid fatty acyltransferase expression vector and Escherichia coli
[0030] According to the known amino acid fatty acyl nucleotide sequence (GenBank: AB610978.1), the gene fragment (SEQ ID NO.1) encoding amino acids 21-619 was obtained by gene synthesis after Jcat codon optimization. MscI and XhoI restriction sites were added to the 5' and 3' ends of the fragment, respectively.
[0031] After the synthetic gene fragment and pET22b vector were digested by MscI and XhoI respectively, the target fragment was recovered by gel, the target fragment was ligated, and the ligated product was transformed into E.coli BL21(DE3), cultured overnight on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com