Synthesis process of sulfonic-group rhodamine compound
A synthesis process and compound technology are applied in the field of synthesis technology of sulforhodamine compounds, which can solve the problems of high price of sulfonyl fluorescein, increase production cost, low synthesis yield and the like, and achieve energy saving in production cost and production safety. Improve the effect of simplifying the operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
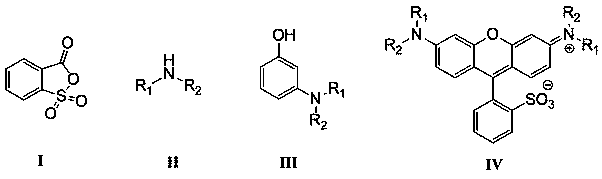
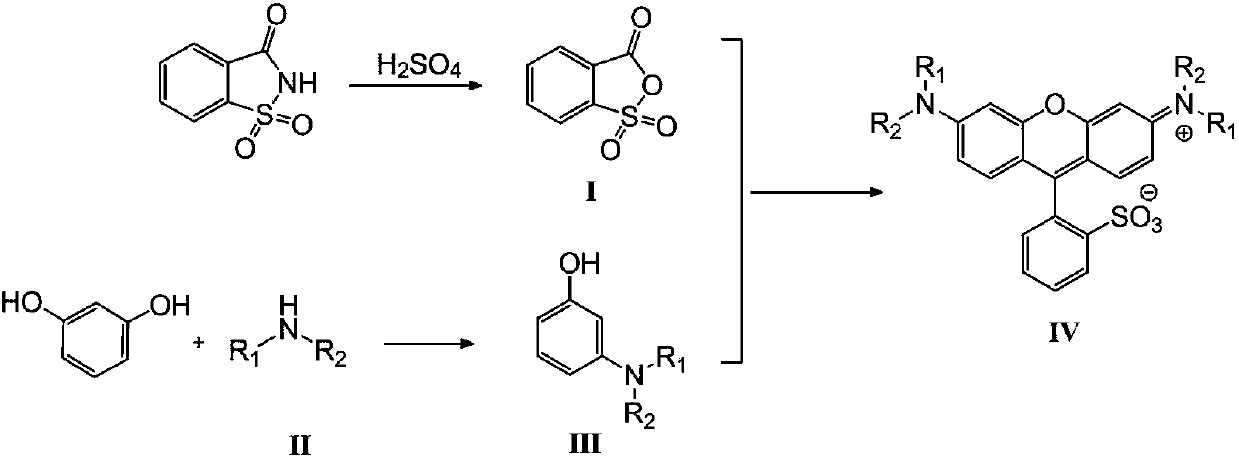
[0028] N 2 Under protection, add 9.0g of saccharin solid and 13mL of concentrated sulfuric acid (concentration: 98%) in sequence in a 50mL three-necked flask, stir and react at 140°C for 2h, then cool to room temperature, a large amount of solids are precipitated in the reaction mixture, filter, and filter the resulting solid Wash with ice water and dry to obtain 7.3 g of o-sulfobenzoic anhydride white solid with a structure represented by formula (I), with a yield of 81%.
[0029] The obtained compound with the structure shown in formula (I) is carried out nuclear magnetic spectrum analysis, and the results are as follows: 1 HNMR (500MHz, CDCl3) δ8.77(d, J=7.3Hz, 1H), 8.05(d, J=7.3Hz, 1H), 7.76(t, J=2.2Hz, 1H), 7.53(t, J= 8.5Hz, 1H).
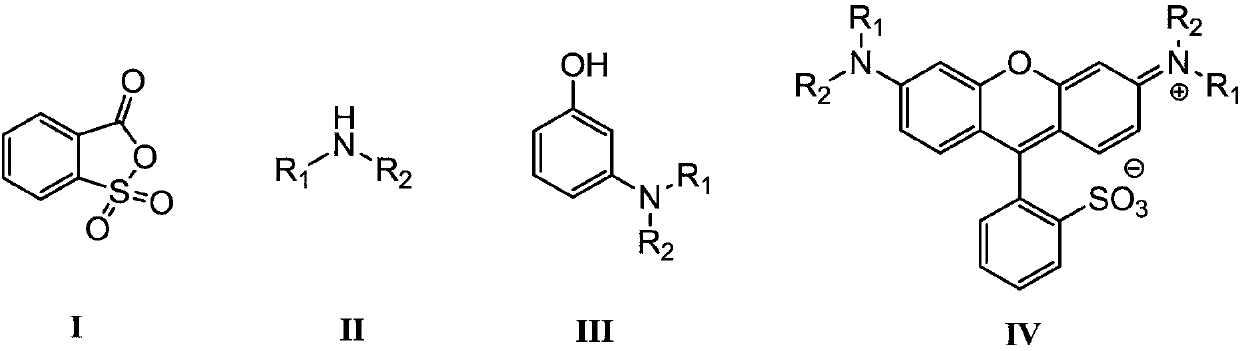
[0030] 4.8g of diethylamine and 0.3mL of phosphoric acid (weight concentration is 85%) are fully mixed in 20mL of toluene, prepared to form a reaction raw material solution; N 2 Under protection, slowly drop the above prepared reaction raw m...
Embodiment 2
[0034] in N 2 Under protection, add 9.0g of saccharin solid and 10.5mL of concentrated sulfuric acid (concentration: 98%) in sequence in a 50mL three-neck flask, stir and react at 140°C for 2h, then cool to room temperature, a large amount of solids are precipitated in the reaction mixture, filter, and filter the obtained The solid was washed with ice water and dried to obtain 6.3 g of o-sulfobenzoic anhydride as a white solid with a yield of 70%.
[0035] 5.4g of diethylamine and 0.4mL of phosphoric acid (weight concentration is 85%) are fully mixed in 25mL of toluene, prepared to form a reaction raw material solution; N 2 Under protection, slowly drop the above prepared reaction raw material solution into a mixture of 9.1g resorcinol and 20mL toluene at a rate of 1 drop per second, heat and reflux at 55°C for 10h, cool to room temperature, and let it stand Separate the layers and separate the organic layer, wash the organic layer with water, and finally spin dry the solvent...
Embodiment 3
[0039] in N 2 Under protection, add 100.0g of saccharin solid and 155mL of concentrated sulfuric acid (concentration: 98%) to a 500mL three-neck flask successively, stir and react at 145°C for 3h, then cool to room temperature, a large amount of solids are precipitated in the reaction mixture, filter, and filter the resulting solid Wash with ice water and dry to obtain 87.5 g of o-sulfobenzoic anhydride as a white solid with a yield of 87%.
[0040] 193.4g of diethylamine and 10.0mL of phosphoric acid (weight concentration is 85%) are fully mixed in 400mL of toluene, prepared to form a reaction raw material solution; N 2 Under protection, slowly drop the above prepared reaction raw material solution into a mixture of 300.0g resorcinol and 600mL toluene at a rate of 1 drop per second, heat the reaction at 50°C for 12h, cool to room temperature, and let stand to separate and the organic layer was separated, the organic layer was washed with water, and finally the solvent was sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com