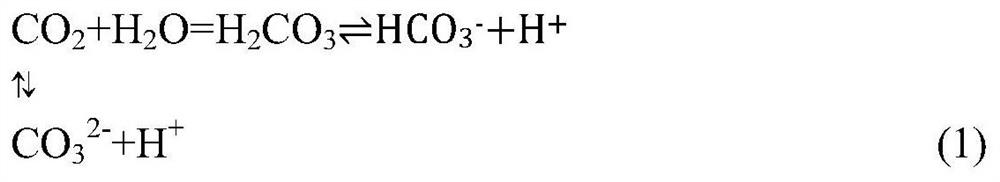A method for producing light calcium carbonate and magnesium sulfate heptahydrate from salt mud
A technology of light calcium carbonate and magnesium sulfate heptahydrate, applied in the direction of magnesium sulfate, chemical instruments and methods, calcium carbonate/strontium/barium, etc., can solve the problems of high cost of hydrochloric acid and sodium hydroxide, low solubility of magnesium bicarbonate, and water volume and high energy consumption of pyrolysis, to achieve the effect of accelerating the carbonization reaction rate, low equipment requirements, and reducing water consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of salt mud produces the method for light calcium carbonate and magnesium sulfate heptahydrate, comprises the following steps:
[0045] Step A: Add calcium sulfate with a ratio of 1:1.05 to the amount of magnesium hydroxide contained in salt mud to a certain quality of salt mud, put it in a 500ml beaker, add water to make a total volume of 400ml, and a solid-to-liquid ratio of 1 :4 Serum.
[0046] Step B: Pass 60ml / min pure carbon dioxide into the slurry obtained in step A, and carbonize at a temperature of 40°C for 60min. Calcium sulfate is converted into calcium carbonate, magnesium hydroxide is converted into soluble magnesium sulfate, and calcium and magnesium are separated by filtration.
[0047] Step C: Dry the filter cake obtained in Step B at 110°C for 10 hours, and then pulverize it to obtain a light calcium carbonate product
[0048] Step D: Concentrate the filtrate obtained in Step B at 100° C., crystallize by cooling, and dry to obtain the magnesium ...
Embodiment 2
[0050] 4. A method for producing light calcium carbonate and magnesium sulfate heptahydrate from salt mud, comprising the following steps:
[0051] Step A: Add calcium sulfate with a ratio of 1:1 to the amount of magnesium hydroxide contained in salt mud to a certain quality of salt mud, put it in a 500ml beaker, add water to make a total volume of 400ml, and a solid-to-liquid ratio of 1 :15 Serum.
[0052] Step B: Introduce 40ml / min pure carbon dioxide into the slurry obtained in step A and carbonize for 20min at a temperature of 60°C. Calcium sulfate is converted into calcium carbonate, magnesium hydroxide is converted into soluble magnesium sulfate, and calcium and magnesium are separated by filtration.
[0053] Step C: Dry the filter cake obtained in Step B at 110° C. for 6 hours, and then pulverize it to obtain a light calcium carbonate product.
[0054] Step D: Concentrate the filtrate obtained in Step B at 105° C., crystallize by cooling, and dry to obtain the magnesium ...
Embodiment 3
[0056] A kind of salt mud produces the method for light calcium carbonate and magnesium sulfate heptahydrate, comprises the following steps:
[0057] Step A: Add calcium sulfate with a ratio of 1:0.95 to the amount of magnesium hydroxide contained in salt mud to a certain quality of salt mud, put it in a 500ml beaker, add water to make a total volume of 400ml, and a solid-to-liquid ratio of 1 :10 Serum.
[0058] Step B: Introduce 60 ml / min of flue gas with a carbon dioxide volume fraction greater than 10% into the slurry obtained in step A, carbonize for 120 min at a temperature of 20° C., convert calcium sulfate into calcium carbonate, and convert magnesium hydroxide into soluble magnesium sulfate. Filtration realizes the separation of calcium and magnesium.
[0059] Step C: Dry the filter cake obtained in Step B at 110°C for 8 hours, and obtain light calcium carbonate product after crushing
[0060] Step D: Concentrate the filtrate obtained in Step B at 110°C, crystallize ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com