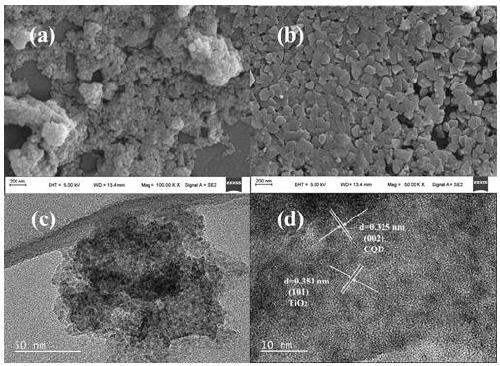
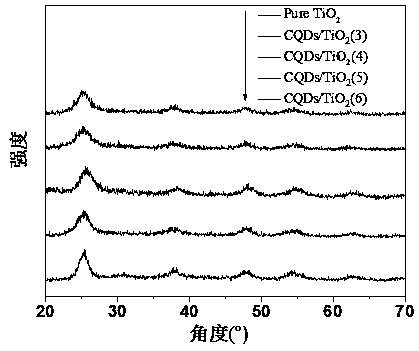
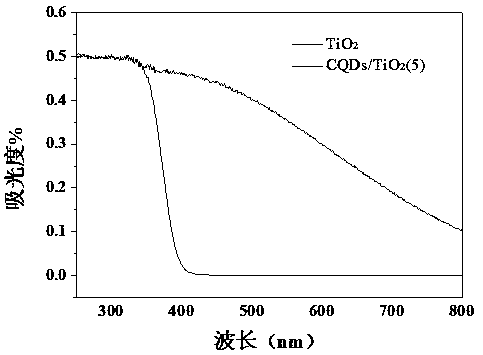
Preparation of carbon quantum dot supported TiO2 nano-composite material, and application thereof in photocatalytic reduction of CO2
A technology of nanocomposite materials and carbon quantum dots, which is applied in the field of photocatalytic reduction of CO2, and can solve problems such as photocatalytic performance limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of CQDs: Take 2.5g of glucose and dissolve it in 10mL of deionized water with stirring, and heat the mixture in a microwave oven on high heat for 5min. After the reaction is completed, viscous orange-yellow CQDs are obtained, and 10mL of deionized water is added to it Diluted into CQDs solution.
[0035] (2) CQDs / TiO 2 Synthesis of nanocomposites: Take 5 mL of the CQDs solution synthesized above, add 50 mL of deionized water, and add 5 mL of tetrabutyl titanate, stir for 1 h, transfer to a 100 mL stainless steel autoclave lined with polytetrafluoroethylene, and control the temperature It was reacted at 110°C for 24 hours; after the reaction, the mixture was cooled to room temperature, washed several times with deionized water, dried in an oven at 60°C for 12 hours, and the dried sample was ground to obtain CQDs / TiO 2 (5) Nanocomposite materials.
[0036] Photochemical performance test: CQDs / TiO 2 (5) CO for photocatalytic reduction 2 , the production r...
Embodiment 2
[0038] (1) Synthesis of CQDs: 2.5g of glucose was fully dissolved in 10mL of deionized water with stirring, and the mixture was heated in a microwave oven on high heat for 5min to obtain viscous orange-yellow CQDs; 10mL of deionized water was added to it to dilute into CQDs solution.
[0039] (2) CQDs / TiO 2 Synthesis of nanocomposites: Take 3mL of the above CQDs solution, add 50mL of deionized water, and add 5mL of tetrabutyl titanate, stir for 1h, transfer the mixture to a 100mL stainless steel autoclave lined with polytetrafluoroethylene, The reaction temperature was controlled at 110°C for 24 hours; after the reaction, the mixture was cooled to room temperature, washed several times with deionized water, dried in an oven at 60°C for 12 hours, and the dried sample was ground to obtain CQDs / TiO 2 (3) Nanocomposite materials.
[0040] Photochemical performance test: CQDs / TiO 2 (3) CO for photocatalytic reduction 2 , the production rate of CO was 5.2 μmol / g.
Embodiment 3
[0042] (1) Synthesis of CQDs: 2.5g of glucose was fully dissolved in 10mL of deionized water with stirring, and the mixture was heated in a microwave oven on high heat for 5min. After the reaction was completed, viscous orange-yellow CQDs were obtained, and 10mL of deionized water was added to it. Diluted into CQDs solution.
[0043] (2) CQDs / TiO 2 Synthesis of nanocomposites: Take 4 mL of the CQDs solution synthesized above, add 50 mL of deionized water, and add 5 mL of tetrabutyl titanate, stir for 1 h, transfer to a 100 mL stainless steel autoclave lined with polytetrafluoroethylene, and control the temperature It was reacted at 110°C for 24 hours; after the reaction, the mixture was cooled to room temperature, washed several times with deionized water, dried in an oven at 60°C for 12 hours, and the dried sample was ground to obtain CQDs / TiO 2 (4) Nanocomposite materials.
[0044] Photochemical performance test: CQDs / TiO 2 CO for photocatalytic reduction 2 , the product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com