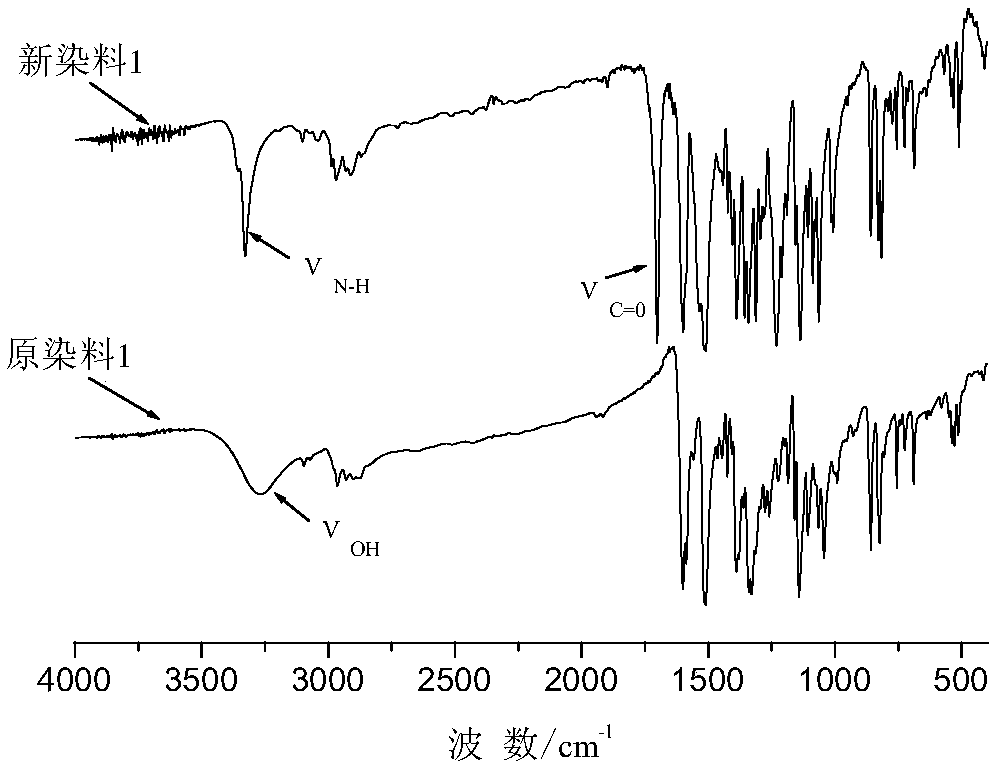
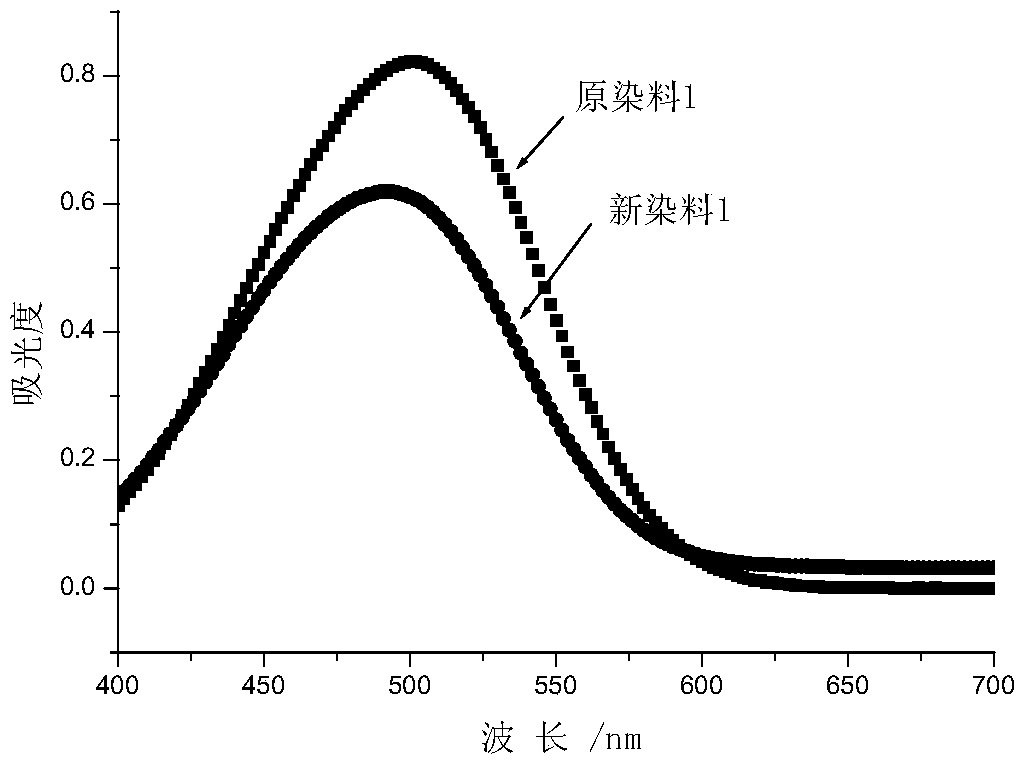
Novel carbamate-containing azo disperse dye and preparation and application thereof
A technology of disperse dyes and carbamic acid, applied in the preparation of azo dyes, azo dyes, organic dyes, etc., can solve the problems of slow fiber diffusion rate, slow dyeing rate, low solubility, etc., to reduce water solubility, Effect of increasing van der Waals forces, increasing molecular volume and molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
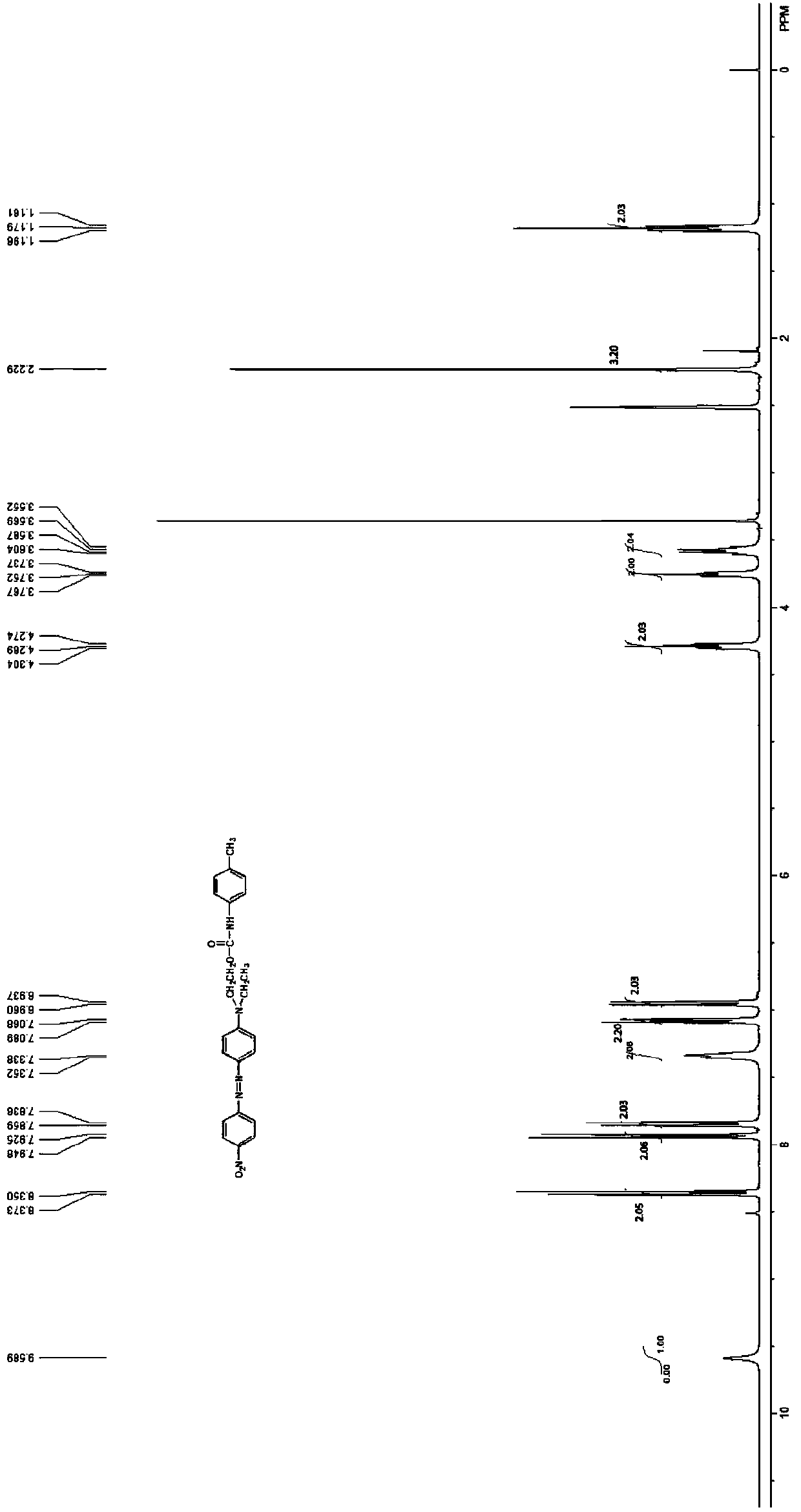
[0036] 1. Molecular structural formula of dye
[0037]
[0038] 2. Reaction equation
[0039]
[0040] 3. Synthesis process:
[0041] Add 6.00g of purified and vacuum-dried raw dye C.I. Disperse Red 1 into a 500ml three-necked flask, add 300ml of dehydrated dichloromethane as a solvent, stir fully to dissolve the disperse dye completely, and then accurately measure 2.90 ml of isocyanate compound, quickly added to the solution and allowed to fully stir to dissolve, then slowly drop 6ml of dehydrated triethylamine into the reactor, and react at room temperature for 3 to 6 hours. TLC spotting to track the reaction process, after the reaction is completed, mix with 100ml of dilute acid solution and extract the liquid to obtain the dye solution, remove the dichloromethane solvent from the dye solution by rotary evaporation, and then add an appropriate amount of 10% sodium carbonate solution Wash and filter, and finally wash with water to obtain 8.10 g of crude product.
[...
Embodiment 2
[0061] (1) Molecular structural formula of dye
[0062]
[0063] (2) Reaction equation
[0064]
[0065] (3) Synthesis process
[0066] Add 4.00g of purified and vacuum-dried raw dye 2 (self-made diazo-coupled dye intermediate) into a 500ml three-necked flask, add 300ml of dehydrated dichloromethane as a solvent, stir fully to dissolve the disperse dye completely , then accurately measure 3.56ml of isocyanate compound, quickly add it into the solution and allow it to fully stir to dissolve, then slowly add 3ml of dehydrated triethylamine dropwise into the reactor, and react at room temperature for 3 to 6 hours. TLC spotting to track the reaction process, after the reaction is completed, mix with 100ml of dilute acid solution and extract the liquid to obtain the dye solution, remove the dichloromethane solvent from the dye solution by rotary evaporation, and then add an appropriate amount of 10% sodium carbonate solution Wash and filter, and finally wash with water to o...
Embodiment 3
[0085] (1) Molecular structural formula of dye
[0086]
[0087] (2) Reaction equation
[0088]
[0089] (3) Synthesis process
[0090]Add 4.00g of purified and vacuum-dried original dye C.I. Disperse Blue 106 to a 500ml three-necked flask, add 300ml of dehydrated dichloromethane as a solvent, stir fully to dissolve the disperse dye completely, and then accurately measure 1.66 ml of isocyanate compound, quickly added to the solution and allowed to fully stir to dissolve, then slowly drop 6ml of dehydrated triethylamine into the reactor, and react at room temperature for 3 to 6 hours. TLC spotting to track the reaction process, after the reaction is completed, mix with 100ml of dilute acid solution and extract the liquid to obtain the dye solution, remove the dichloromethane solvent from the dye solution by rotary evaporation, and then add an appropriate amount of 10% sodium carbonate solution Wash and filter, and finally wash with water to obtain 5.49 g of crude produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar extinction coefficient | aaaaa | aaaaa |
| Molar extinction coefficient | aaaaa | aaaaa |
| Molar extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com