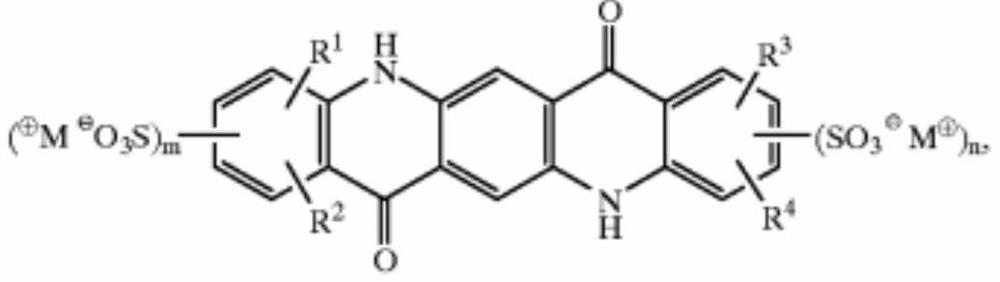
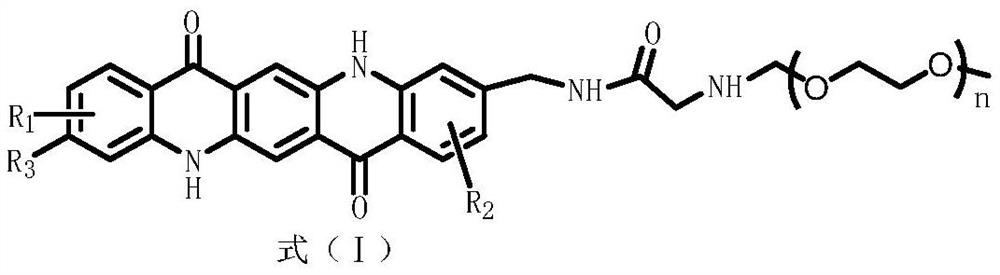
A pyrine ketone derivatives and their preparation methods and applications
A technology of quinacridone and derivatives, which is applied in the field of quinacridone derivatives and their preparation, can solve the problems of difficult preparation of nano-scale color paste, poor stability of color paste, poor pigment dispersion, etc., and achieve the change of molecular polarity , high dispersion stability, and the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
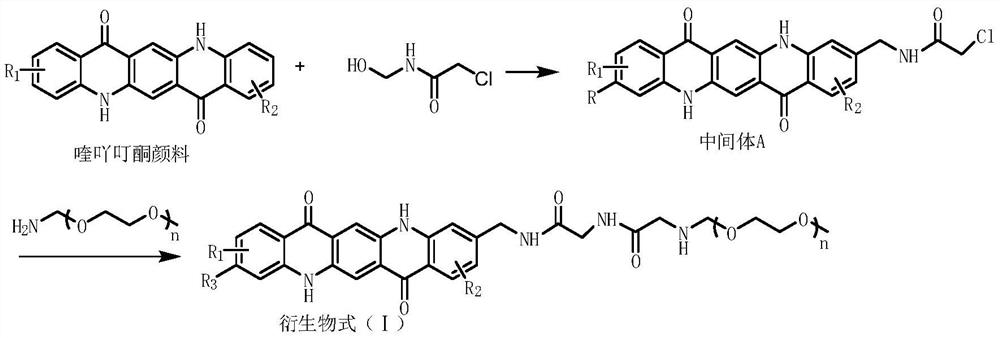
[0038] The synthesis of quinacridone derivative 1, its synthetic equation is as follows:
[0039]
[0040] Add 240g of concentrated sulfuric acid into a 500ml four-neck flask, cool to 0-5°C, add 34g (0.1mol) Pigment Red 122, 12.3g (0.1mol) N-hydroxyethyl chloroacetamide in batches, ℃ for 12 hours, pour the mixture into 1000ml distilled water, filter, wash the filter cake with water until neutral, and obtain 40g of red solid powder after drying.
[0041] Add 40 g (0.09 mol) of the above solid mixture into 200 ml ethanol, add 94.5 g (0.09 mol, n=15) of polyetheramine, reflux for 4 h, distill off ethanol under reduced pressure to obtain 131 g of red solid.
Embodiment 2
[0043]The synthesis of quinacridone derivative 2, its synthetic equation is as follows:
[0044]
[0045] Add 240g of concentrated sulfuric acid into a 500ml four-necked flask, cool to 0-5°C, add 34g (0.1mol) of Pigment Red 122 in batches, and then add 24.6g (0.2mol) of N-hydroxyethyl chloroacetamide. -5°C, heat preservation reaction for 12 hours, pour the mixture into 1000ml of distilled water, filter, wash the filter cake with water until neutral, and obtain 42.8g of red solid powder after drying.
[0046] Add 42.8 g (0.08 mol) of the above solid mixture into 200 ml of ethanol, add 189 g (0.18 mol, n=15) of polyetheramine, reflux for 4 hours, distill off ethanol under reduced pressure to obtain 198 g of red solid.
Embodiment 3
[0048] The synthesis of quinacridone derivative 3, its synthetic equation is as follows:
[0049]
[0050] Add 240g of concentrated sulfuric acid into a 500ml four-necked flask, cool to 0-5°C, add 38g (0.1mol) of Pigment Violet 19 in batches, and then add 12.3g (0.1mol) of N-hydroxyethyl chloroacetamide. -5°C, heat preservation reaction for 12 hours, pour the mixture into 1000ml of distilled water, filter, wash the filter cake with water until neutral, and obtain 36.3g of red solid powder after drying.
[0051] Add 36.3 g (0.075 mol) of the above solid mixture into 200 ml of ethanol, then add 78.75 g (0.075 mol, n=15) of polyetheramine, reflux for 4 hours, distill off ethanol under reduced pressure to obtain 102 g of red solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com