Enhanced gene delivery methods
A technology of gene delivery and target cells, applied in other methods of inserting foreign genetic materials, biochemical equipment and methods, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Effect of E4ORF1+ co-culture on HSPC expansion and virus transduction
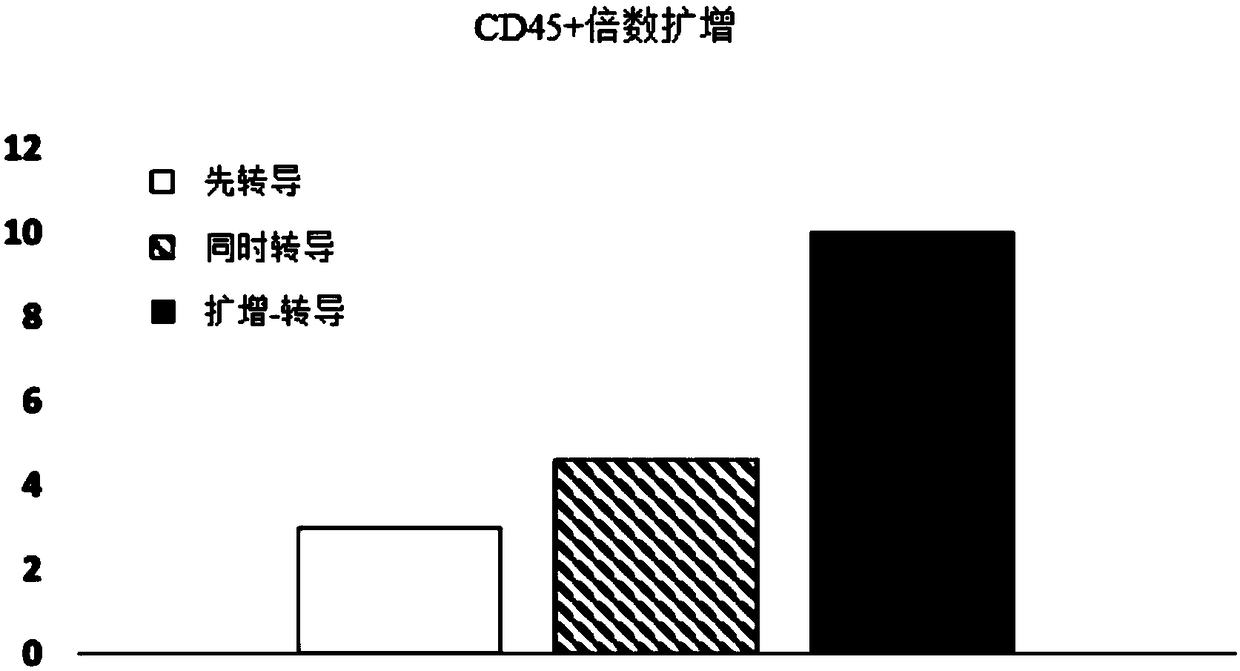
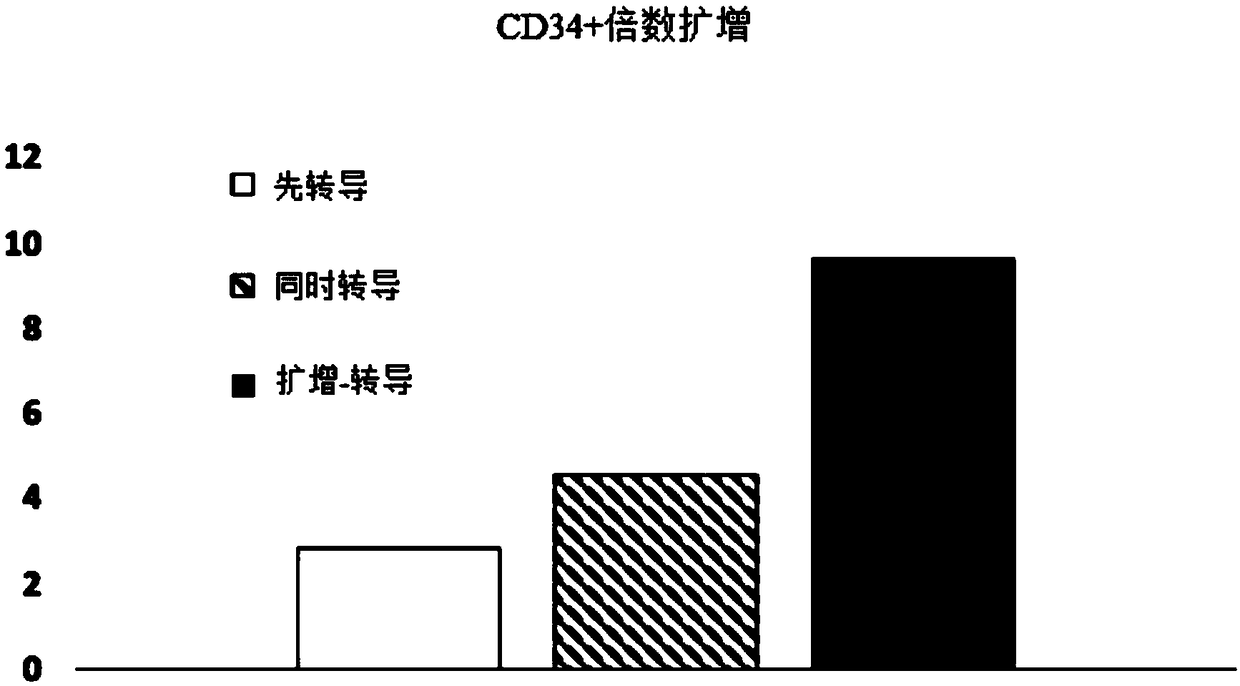
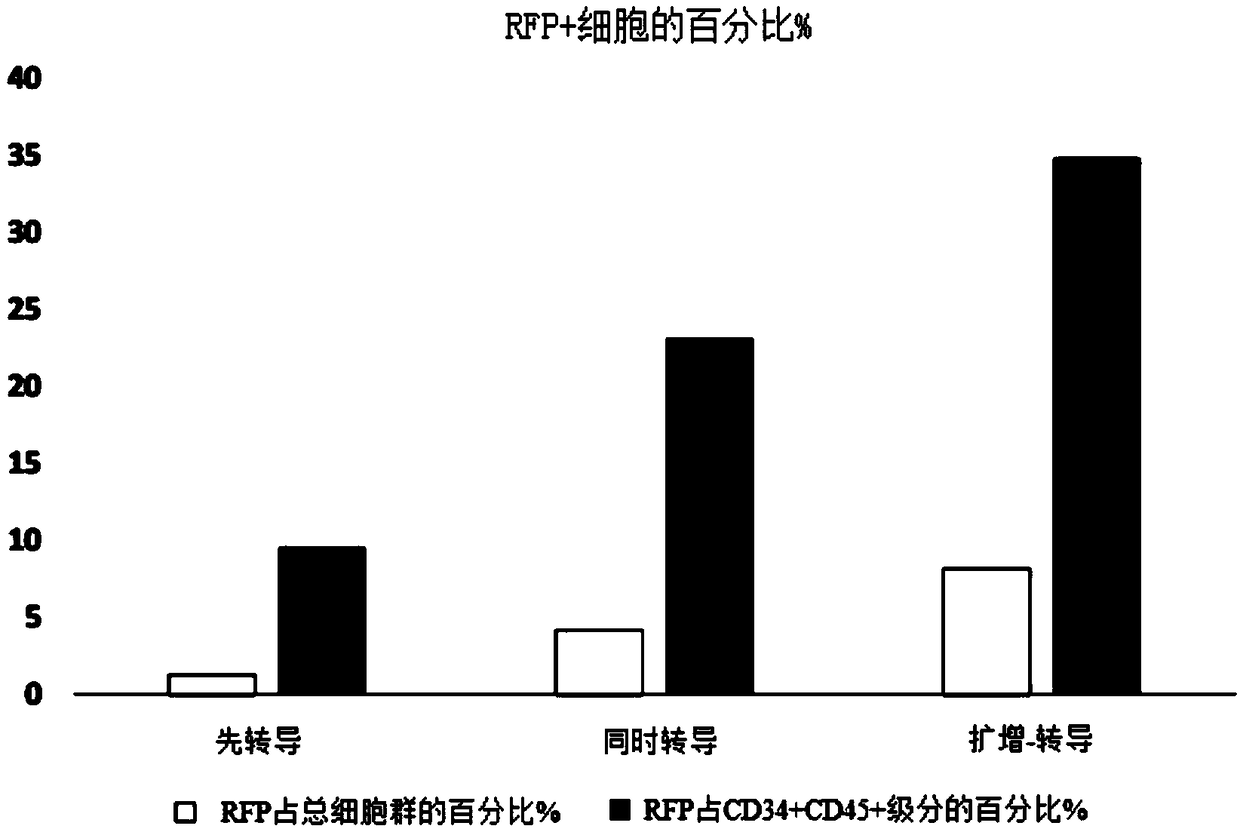
[0085] Experiments were performed to assess the effect of E4ORF1+ endothelial cells on the ability to expand and transduce CD34+ / CD45+ HSPCs. CD34+ cells (Lonza) were transduced with red fluorescent protein (RFP) and expanded on E4ORF1+ umbilical vein endothelial cell (UVEC) cultures in 6-well plates according to three different protocols as follows:
[0086] In protocol 1, transduction was initiated 24 hours before initiation of HSPC-E4ORF1+UVEC co-culture ("transduction first").
[0087] In protocol 2, transduction was performed while co-cultivating with HSPC-E4ORF1+UVEC (simultaneous transduction).
[0088] In protocol 3, HSPCs were co-cultured with E4ORF1+UVECs for 48 hours, after which the floating HSPCs were transferred to empty wells for transduction (24 hours), after transduction, HSPCs were then co-cultured with E4ORF1+UVECs ("amplification-transduction").
[0089] In each of the above...
Embodiment 2
[0092] Suspension Fraction & Cytokines Effect on Viral Transduction Efficiency
[0093] Experiments were performed to determine the effect of removal of the "floating fraction" of CD34+ hematopoietic cells on transduction efficiency, and to determine whether cytokine concentration had an effect on transduction efficiency.
[0094] CD34+ cells (Lonza) were transduced with blue fluorescent protein (BFP) and expanded on E4ORF1+UVEC cultures in 6-well plates according to four different protocols as follows:
[0095] In protocols 1 and 3, HSPCs were expanded on E4ORF1+UVEC cultures for 96 hours and subsequently transduced by incubating the HSPCs with a lentivirus engineered to express BFP for 24 hours, while the HSPCs were still on the E4ORF1+EC feeder layer superior. Then, HSPCs were expanded for two more days in E4ORF1+EC cultures. The results obtained using these protocols are referred to in the figure as "co-transduction" results.
[0096] In protocols 2 and 4, HSPCs were ...
Embodiment 3
[0102] Rescue of transfected cells by subsequent EC co-culture
[0103] Transfected or transduced cells, eg, cells transfected by electroporation, can be cultured with ECs after transfection or transduction. This subsequent co-cultivation can "rescue" cells that were destroyed during transfection or transduction - reducing death and loss of transfected or transduced cells. This co-cultivation should start "immediately" (as defined in this patent specification) after the start of the transfection or transduction step, and should continue for a sufficient time to allow recovery of the target cells. Without wishing to be bound by theory, it is believed that such "rescue" may be the result of cell-cell contact between the two cell populations (i.e. transduced / transfected cells and EC), and / or may be the result of transducing Exposure of transfected cells results in soluble factors secreted by ECs, such as angiogenic factors and cytokines. Co-cultivation of the two cell populat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com