A kind of thiomacrolide compound and its preparation method and anti-aquaculture disease fungus activity application
A technology of macrolides and macrolides, which is applied in the field of microbial medicine and medicinal chemistry, and can solve problems such as no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
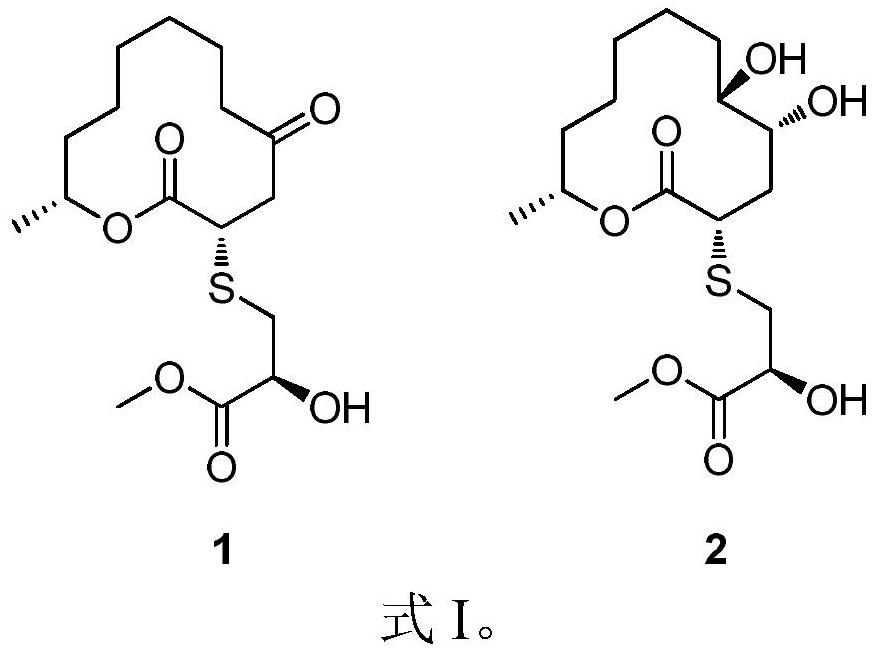
[0024] Embodiment 1. Fermentative production and separation and purification of compounds 1 and 2 shown in formula I:
[0025] Cladosporium cladosporioides obtained according to the above-mentioned literature or public circulation channels was purified and inoculated on a PDA plate medium, and cultured in an incubator at 28° C. for 6 days.
[0026] An appropriate amount of bacteria on the surface of the above-mentioned PDA plate was cut, inoculated into a sterilized Erlenmeyer flask filled with solid medium, left to stand at room temperature for 48 days, and then inactivated with ethyl acetate for use.
[0027] The solid medium contains 70g of rice, 0.3g of peptone, 0.1g of corn steep liquor and 0.1% of methionine per 100mL of natural seawater.
[0028] The product obtained by fermenting and culturing the above-mentioned Cladosporium cladoides on a solid medium was ultrasonically extracted three times with ethyl acetate, and the extracts were combined and concentrated to obtai...
Embodiment 2
[0039] Embodiment 2. Aquatic pathogen inhibitory activity:
[0040] The antibacterial activity of compounds 1 and 2 represented by formula I was detected by the minimum inhibitory concentration method. Select the following two strains of aquatic disease pathogens: Edwardsiella tarda and Edwardsiella ictarda for antibacterial activity test.
[0041] 1) Antibacterial activity test (MIC method):
[0042]Minimum Inhibitory Concentration (MIC), the lowest concentration of a drug that can inhibit bacterial growth in vitro. In a 96 microwell plate, by adding different concentrations of drugs into the bacterial suspension of the bacteria to be tested, observe after incubation, if the indicator bacteria grow in a certain well, it means that the drug concentration in the well cannot inhibit the growth of the bacteria, The liquid in the hole is turbid, and the light transmittance drops obviously. On the contrary, the liquid in the hole is clear, and the decrease in light transmittance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com